Final ID: MP2736
ZEB1 Regulates Coronary Disease Risk Through Epigenetic Control of Smooth Muscle Cell Identity
Abstract Body (Do not enter title and authors here): Background: Vascular smooth muscle cells (SMCs) contribute significantly to heritable coronary artery disease (CAD) risk and undergo phenotypic transitions in the intimal plaque during atherosclerosis. ZEB1 is a master regulator of epithelial-to-mesenchymal transition, that has been associated with CAD through human genetic studies. ZEB1 orchestrates cell state changes through modulation of TGFβ signaling and numerous epigenetic regulators. However, the mechanisms underlying the genetic association between ZEB1 and CAD remain unexplored.
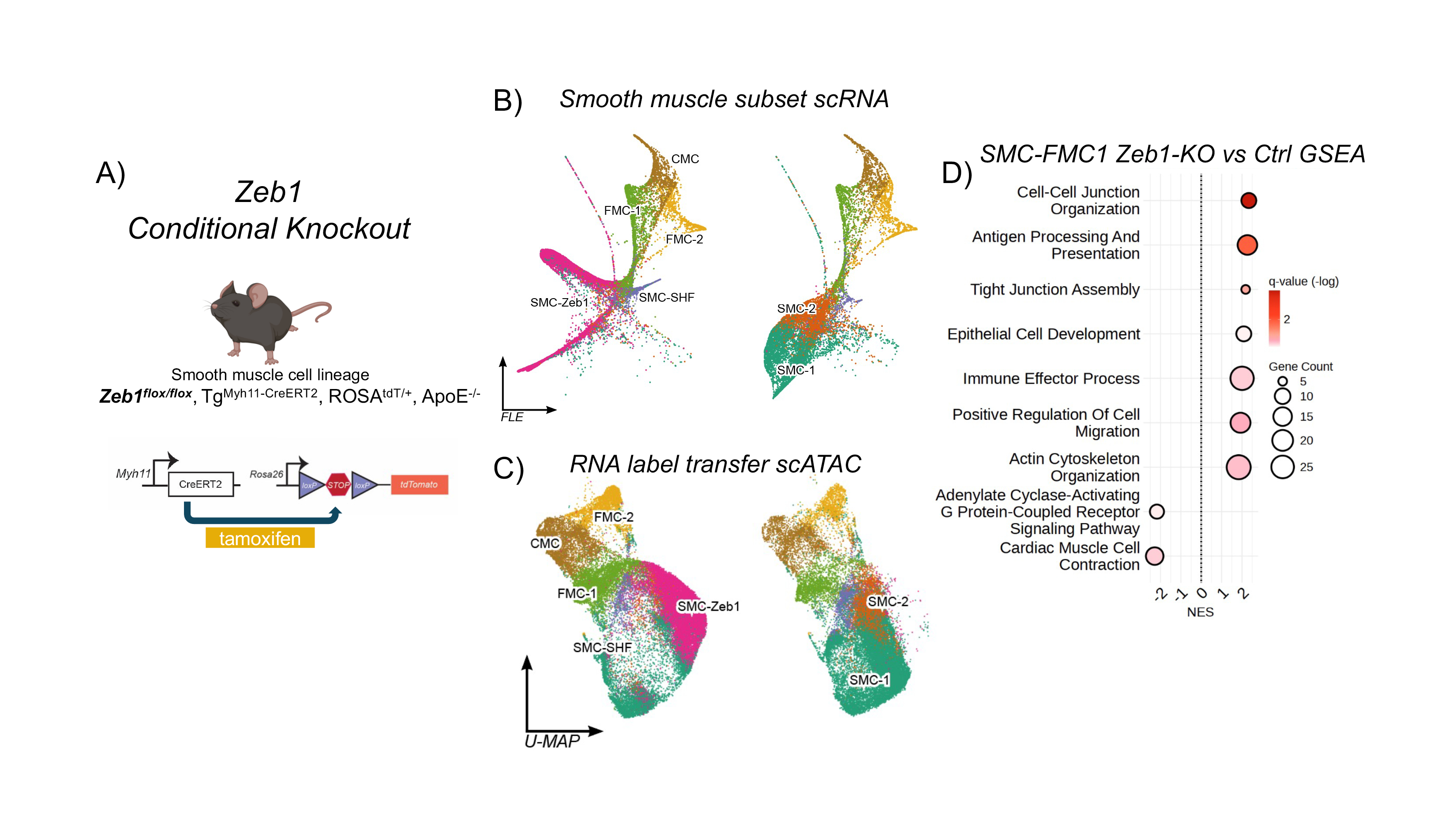
Methods: Single-cell RNA and ATAC sequencing on lineage-traced SMCs from the atherosclerotic aortic roots of SMC-specific Zeb1 knockout mice (Zeb1-KO; Myh11CreERT2, ROSAtdT/+, ApoE-/-) were collected after 16 weeks on high-fat diet to characterize the changes in transcriptomic and epigenetic landscape. siRNA knockdown of ZEB1 in combination with TGFβ and IFNγ stimulations were performed in vitro on human coronary artery SMCs (HCASMCs) to validate in vivo findings.
Results: Zeb1-KO single cell RNA and ATAC sequencing showed the emergence of a novel epithelial-like SMC state characterized by robust ectopic activation of tricellular tight junction and cell polarity genes. Despite this increase in epithelial features, Zeb1-KO also led to an increase in phenotypically transitioning SMCs contributing to the intimal plaque, accompanied by multi-fold enrichment in interferon-gamma (IFNγ) signaling targets. Motif analysis revealed near complete opening of chromatin accessibility at previously closed ZEB1 binding motifs, implicating ZEB1 mediated epigenetic repression. In vitro co-stimulation with TGFβ and IFNγ showed that siZEB1 abolished the inhibitory effect of TGFβ on IFNγ signaling and demonstrated enriched for migratory and growth factor response functions by bulk RNAseq. Cell-cell signaling analysis by MultiNicheNet identified ectopic expression of migratory marker Lamc2 and concurrent increases in its interacting integrin partners in Zeb1-KO SMCs. Furthermore, siZEB1 HCASMCs also showed elevated expression of LAMC2, its integrin interacting partners, as well as increased wound healing capacity by scratch assay.
Conclusion: We identify ZEB1 as a critical epigenetic repressor required for the maintenance of the SMC cell state, suppressing epithelial marker expression and modifying cellular response to interferon signaling to ultimately augment SMC phenotypic transitions, linking genetic association between ZEB1 and CAD risk.
Methods: Single-cell RNA and ATAC sequencing on lineage-traced SMCs from the atherosclerotic aortic roots of SMC-specific Zeb1 knockout mice (Zeb1-KO; Myh11CreERT2, ROSAtdT/+, ApoE-/-) were collected after 16 weeks on high-fat diet to characterize the changes in transcriptomic and epigenetic landscape. siRNA knockdown of ZEB1 in combination with TGFβ and IFNγ stimulations were performed in vitro on human coronary artery SMCs (HCASMCs) to validate in vivo findings.
Results: Zeb1-KO single cell RNA and ATAC sequencing showed the emergence of a novel epithelial-like SMC state characterized by robust ectopic activation of tricellular tight junction and cell polarity genes. Despite this increase in epithelial features, Zeb1-KO also led to an increase in phenotypically transitioning SMCs contributing to the intimal plaque, accompanied by multi-fold enrichment in interferon-gamma (IFNγ) signaling targets. Motif analysis revealed near complete opening of chromatin accessibility at previously closed ZEB1 binding motifs, implicating ZEB1 mediated epigenetic repression. In vitro co-stimulation with TGFβ and IFNγ showed that siZEB1 abolished the inhibitory effect of TGFβ on IFNγ signaling and demonstrated enriched for migratory and growth factor response functions by bulk RNAseq. Cell-cell signaling analysis by MultiNicheNet identified ectopic expression of migratory marker Lamc2 and concurrent increases in its interacting integrin partners in Zeb1-KO SMCs. Furthermore, siZEB1 HCASMCs also showed elevated expression of LAMC2, its integrin interacting partners, as well as increased wound healing capacity by scratch assay.
Conclusion: We identify ZEB1 as a critical epigenetic repressor required for the maintenance of the SMC cell state, suppressing epithelial marker expression and modifying cellular response to interferon signaling to ultimately augment SMC phenotypic transitions, linking genetic association between ZEB1 and CAD risk.
More abstracts on this topic:
ACTIVATION AND TARGETABILITY OF TYMP-IL-6-TF AXIS IN THE SKIN MICROENVIRONMENT IN UREMIC CALCIPHYLAXIS
Lotfollahzadeh Saran, Chitalia Vipul
A single-cell lung atlas of human pulmonary arterial hypertensionDai Zhiyu, Yi Dan, Zhao Hanqiu, Hong Jason, Fallon Michael