Final ID: MP369
Emergence of Hypertension in Thyroid Cancer Patients on BRAF ± MEK Inhibitors: A Closer Look at Incidence and Contributing Risk Factors
Abstract Body (Do not enter title and authors here): Background: Inhibitors of BRAF (BRAFi) and MEK (MEKi) are often used in advanced BRAFV600E-mutated thyroid cancer (BRAFm-TC), and at least one drug combination is FDA-approved. However, cardiovascular (CV) adverse effects, including hypertension (HTN), are increasingly recognized.
Research Question: We examined the incidence of new or worsening HTN during treatment with BRAFi±MEKi and the associated baseline risk factors in patients with BRAFm-TC.
Methods: This single-center retrospective cohort study included patients with BRAFm-TC treated with therapeutic-intent BRAFi±MEKi from Jan 2016-Mar 2024. Development of HTN was defined by initiation of new antihypertensives in patients without a history of HTN, up-titration of existing or addition of new antihypertensives in pre-existing HTN, or documentation of elevated blood pressures (BP≥140/90) in notes.
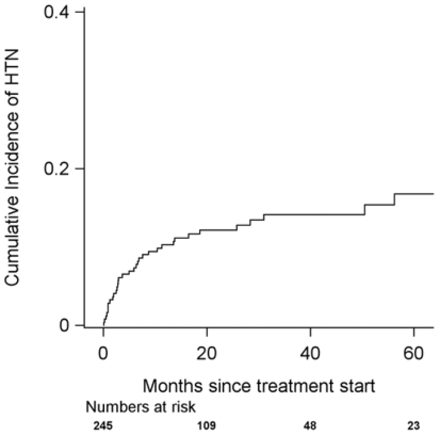
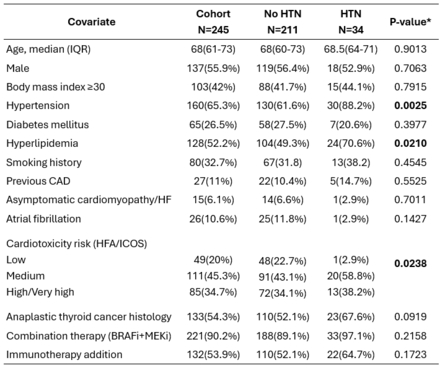
Results: Among 245 patients with a median follow-up of 35.9 months (95%CI 31.4-41.2) (Table 1), new or worsening HTN occurred in 34 patients (13.9%) (Fig. 1). Four patients (1.6%) were diagnosed with new HTN at a median of 6.13 months, and 30 (12.2%) with worsening of pre-existing HTN at a median of 5.44 months. Univariate predictors of HTN risk were anaplastic thyroid cancer histology, history of asymptomatic coronary artery disease (CAD), pre-existing HTN, hyperlipidemia, and moderate to high/very high cardiotoxicity risk by the HFA/ICOS risk score. On multivariable analysis, history of asymptomatic CAD (HR 9.23, 95%CI 2.19–38.87; sHR 8.96, 95%CI 2.94–27.26) and pre-existing HTN (HR 4.38, 95%CI 1.54–12.44; sHR 4.47, 95%CI 1.61–12.37) remained associated with HTN development. During follow-up, HTN led to dose adjustments or switching of BRAF/MEKi in two and one patients, respectively, and treatment interruption or early discontinuation due to BRAF/MEKi-associated HTN occurred in one patient. Amongst 34 patients with incident HTN, 11 discontinued the medication for various reasons throughout the study; in 91% of these cases, HTN was reversible. Of the 23 patients who remained on BRAF/MEKi therapy, HTN was effectively controlled with antihypertensive therapy.
Conclusion: New or worsening HTN was noted in one in eight individuals with BRAFm-TC after initiation of BRAFi±MEKi. Pre-existing HTN and asymptomatic CAD were independently associated with the risk of developing or worsening HTN, highlighting the need for baseline CV risk assessment and close BP monitoring during treatment to allow safer use of these agents.
Research Question: We examined the incidence of new or worsening HTN during treatment with BRAFi±MEKi and the associated baseline risk factors in patients with BRAFm-TC.
Methods: This single-center retrospective cohort study included patients with BRAFm-TC treated with therapeutic-intent BRAFi±MEKi from Jan 2016-Mar 2024. Development of HTN was defined by initiation of new antihypertensives in patients without a history of HTN, up-titration of existing or addition of new antihypertensives in pre-existing HTN, or documentation of elevated blood pressures (BP≥140/90) in notes.
Results: Among 245 patients with a median follow-up of 35.9 months (95%CI 31.4-41.2) (Table 1), new or worsening HTN occurred in 34 patients (13.9%) (Fig. 1). Four patients (1.6%) were diagnosed with new HTN at a median of 6.13 months, and 30 (12.2%) with worsening of pre-existing HTN at a median of 5.44 months. Univariate predictors of HTN risk were anaplastic thyroid cancer histology, history of asymptomatic coronary artery disease (CAD), pre-existing HTN, hyperlipidemia, and moderate to high/very high cardiotoxicity risk by the HFA/ICOS risk score. On multivariable analysis, history of asymptomatic CAD (HR 9.23, 95%CI 2.19–38.87; sHR 8.96, 95%CI 2.94–27.26) and pre-existing HTN (HR 4.38, 95%CI 1.54–12.44; sHR 4.47, 95%CI 1.61–12.37) remained associated with HTN development. During follow-up, HTN led to dose adjustments or switching of BRAF/MEKi in two and one patients, respectively, and treatment interruption or early discontinuation due to BRAF/MEKi-associated HTN occurred in one patient. Amongst 34 patients with incident HTN, 11 discontinued the medication for various reasons throughout the study; in 91% of these cases, HTN was reversible. Of the 23 patients who remained on BRAF/MEKi therapy, HTN was effectively controlled with antihypertensive therapy.
Conclusion: New or worsening HTN was noted in one in eight individuals with BRAFm-TC after initiation of BRAFi±MEKi. Pre-existing HTN and asymptomatic CAD were independently associated with the risk of developing or worsening HTN, highlighting the need for baseline CV risk assessment and close BP monitoring during treatment to allow safer use of these agents.
More abstracts on this topic:
A Curious Complete Heart Block with Carfilzomib
Shah Mohammed, Rahman Naveed, Al-mohamad Talal, Batra Sejal, Vyas Apurva
A Comparison Between Global Longitudinal Strain (GLS) Derived with CMR Feature-Tracking (CMR-FT) and 2D Speckle-Tracking Echocardiography (2D-STE) to Monitor Cancer Therapy-Related Cardiac Dysfunction (CTRCD)Kar Julia, Cohen Michael, Revere Cherie, Mcquiston Samuel, Malozzi Christopher