Final ID: MP2405
Neutrophil Elastase: A Novel Therapeutic Target for Heart Failure with Preserved Ejection Fraction
Abstract Body (Do not enter title and authors here): Introduction: Heart failure with preserved ejection fraction (HFpEF) is a multifarious syndrome, accounting for over half of clinical heart failure patients. Neutrophil elastase (NE) has been recently shown to have a detrimental role in multiple vascular diseases. However, little is known about the functional involvement of NE in HFpEF. Herein, we aimed to explore a causal role of NE in HFpEF.
Methods: Mice underwent a ‘Two-hit’ protocol (high-fat diet and Nω-nitro-L-arginine methyl-ester) for 5 and 15 weeks to induce HFpEF. NE-deficiency mice, pharmacologic inhibitor GW311616A, bone marrow transplantation, and adeno-associated virus-9 (AAV9)-mediated in vivo cardiac-specific gene transfer were applied to explore a causal role for NE and associated target gene in HFpEF pathogenesis. Multiple functional and biochemical analyses were conducted to unravel the underlying molecular mechanisms of NE in HFpEF.
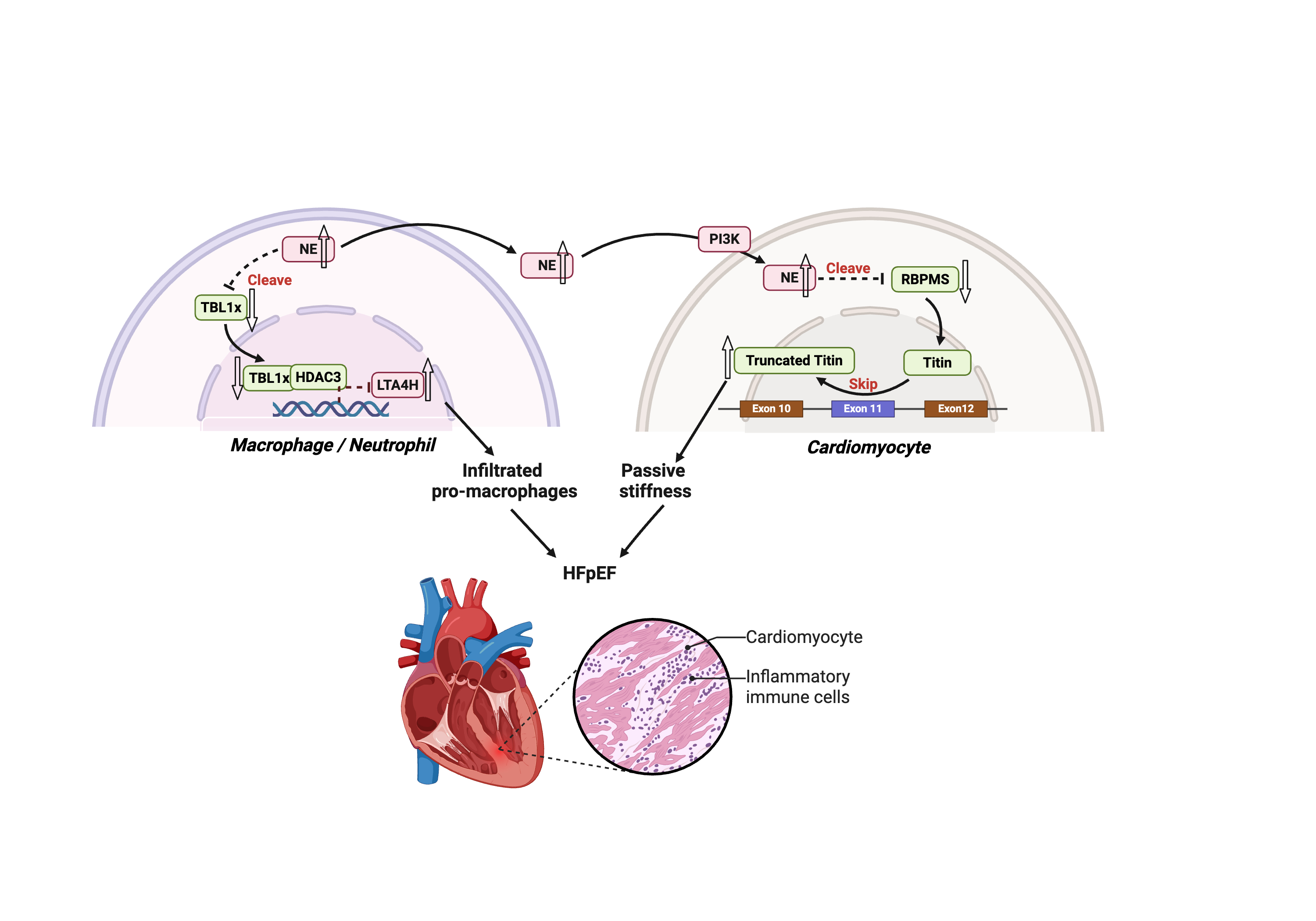
Results: Mice subjected to ‘Two-hit’ protocol displayed significant increases of neutrophil, macrophages, and inflammatory monocytes in bone marrow, spleen, blood and cardiac tissues. NE cardiac gene expression and protein activity were significantly increased during ‘Two-hit’-induced HFpEF. Data showed that cardiomyocytes could uptake NE produced and secreted from infiltrated inflammatory cells. NE deficiency alleviated ‘Two-hit’-induced diastolic dysfunctions at both 5 and 15 weeks in both male and female mice, and reduced cardiac infiltration of inflammatory cells. Mechanistic data showed that NE mediated cardiac infiltration of inflammatory cells through modulating TBL1x-LTA4H signal axis. Moreover, data from bone marrow transplantation confirmed the major contribution of bone marrow-derived NE to HFpEF. Mechanistically, RNA-binding protein with multiple splicing (RBPMS) has been identified as a novel substrate of NE in HFpEF. Further mechanistic studies revealed that NE inhibited alternative splicing of Titin mRNA through downregulating RBPMS in the context of HFpEF. Functionally, AAV9-mediated RBPMS cardiac overexpression could significantly alleviates ‘Two-hit’-induced diastolic dysfunctions. Importantly, AAV9-mediated RBPMS cardiac knockdown could abolish the beneficial effects of NE deficiency on HFpEF phenotype. Finally, administration of NE pharmacological inhibitor significantly ameliorated HFpEF phenotypes.
Conclusion: Our findings suggest that NE-RBPMS-Titin signal axis represents a valuable therapeutic for treating patients with HFpEF.
Methods: Mice underwent a ‘Two-hit’ protocol (high-fat diet and Nω-nitro-L-arginine methyl-ester) for 5 and 15 weeks to induce HFpEF. NE-deficiency mice, pharmacologic inhibitor GW311616A, bone marrow transplantation, and adeno-associated virus-9 (AAV9)-mediated in vivo cardiac-specific gene transfer were applied to explore a causal role for NE and associated target gene in HFpEF pathogenesis. Multiple functional and biochemical analyses were conducted to unravel the underlying molecular mechanisms of NE in HFpEF.
Results: Mice subjected to ‘Two-hit’ protocol displayed significant increases of neutrophil, macrophages, and inflammatory monocytes in bone marrow, spleen, blood and cardiac tissues. NE cardiac gene expression and protein activity were significantly increased during ‘Two-hit’-induced HFpEF. Data showed that cardiomyocytes could uptake NE produced and secreted from infiltrated inflammatory cells. NE deficiency alleviated ‘Two-hit’-induced diastolic dysfunctions at both 5 and 15 weeks in both male and female mice, and reduced cardiac infiltration of inflammatory cells. Mechanistic data showed that NE mediated cardiac infiltration of inflammatory cells through modulating TBL1x-LTA4H signal axis. Moreover, data from bone marrow transplantation confirmed the major contribution of bone marrow-derived NE to HFpEF. Mechanistically, RNA-binding protein with multiple splicing (RBPMS) has been identified as a novel substrate of NE in HFpEF. Further mechanistic studies revealed that NE inhibited alternative splicing of Titin mRNA through downregulating RBPMS in the context of HFpEF. Functionally, AAV9-mediated RBPMS cardiac overexpression could significantly alleviates ‘Two-hit’-induced diastolic dysfunctions. Importantly, AAV9-mediated RBPMS cardiac knockdown could abolish the beneficial effects of NE deficiency on HFpEF phenotype. Finally, administration of NE pharmacological inhibitor significantly ameliorated HFpEF phenotypes.
Conclusion: Our findings suggest that NE-RBPMS-Titin signal axis represents a valuable therapeutic for treating patients with HFpEF.
More abstracts on this topic:
A mechanism whereby SGLT2 inhibitor dapagliflozin reverses cardiac diastolic dysfunction in a model of HFpEF
Liu Man, Liu Hong, Kang Gyeoung-jin, Kim Eunji, Neumann Mitchell, Johnson Madeline, Murikinati Ruthvika, Dudley Samuel
AAV9-Mediated Overexpression of Mixed Lineage Kinase 3 After Transaortic Constriction Improves Left Ventricular FunctionPande Suchita, Martin Gregory, Blanton Robert