Final ID: MP2540
Enrollment Trends and Demographic Representation in Valvular Heart Disease Interventional Trials from 2000 to 2024: A Systematic Review
Abstract Body (Do not enter title and authors here): Introduction: Valvular heart disease (VHD) poses a growing global burden, particularly in aging populations. While transcatheter interventions have expanded rapidly over the past two decades, the extent to which trial populations reflect real-world demographics—especially age and sex—is unclear. Understanding enrollment trends and representation gaps is essential to ensure equitable, generalizable evidence for clinical decision-making.
Methods: We systematically reviewed VHD interventional trials (2000–2024) indexed in PubMed. Surgical and percutaneous trials across aortic, mitral, tricuspid, and pulmonic valves were included. Trial characteristics, demographics, and interventions were analyzed using descriptive and nonparametric statistics.
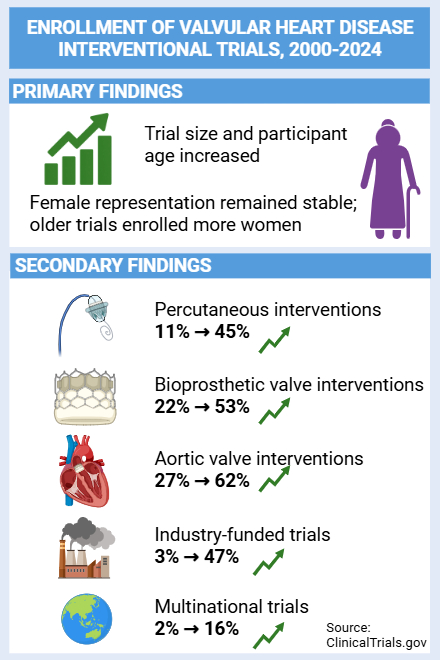
Results: We included 487 trials enrolling 87,412 participants (45% women). Median trial size increased over time (z = 4.98, p < 0.01), and the mean age of participants also rose significantly (z = 10.08, p < 0.01). Trials involving percutaneous (11% to 45%), bioprosthetic (22% to 53%), aortic valve (27% to 62%), and multinational (2% to 16%) interventions increased over time. Older participants were more commonly enrolled in percutaneous, industry-funded, multinational, bioprosthetic, and aortic valve trials. Female representation remained unchanged across time (median 47%, z = -0.48, p = 0.63), but trials enrolling older participants tended to include more women (ρ = 0.196, p < 0.01). Female representation was highest in mitral (59%) and tricuspid (59%) valve trials, and more common in percutaneous (52%) than surgical (42%) interventions.
Conclusion: Over the past two decades, VHD trials have expanded in size and geographic scope, with increased enrollment of older adults, particularly in percutaneous and industry-sponsored studies. However, sex disparities and demographic variability persist. Future trials should prioritize inclusive designs to ensure equitable, generalizable evidence for evolving valve interventions.
Methods: We systematically reviewed VHD interventional trials (2000–2024) indexed in PubMed. Surgical and percutaneous trials across aortic, mitral, tricuspid, and pulmonic valves were included. Trial characteristics, demographics, and interventions were analyzed using descriptive and nonparametric statistics.
Results: We included 487 trials enrolling 87,412 participants (45% women). Median trial size increased over time (z = 4.98, p < 0.01), and the mean age of participants also rose significantly (z = 10.08, p < 0.01). Trials involving percutaneous (11% to 45%), bioprosthetic (22% to 53%), aortic valve (27% to 62%), and multinational (2% to 16%) interventions increased over time. Older participants were more commonly enrolled in percutaneous, industry-funded, multinational, bioprosthetic, and aortic valve trials. Female representation remained unchanged across time (median 47%, z = -0.48, p = 0.63), but trials enrolling older participants tended to include more women (ρ = 0.196, p < 0.01). Female representation was highest in mitral (59%) and tricuspid (59%) valve trials, and more common in percutaneous (52%) than surgical (42%) interventions.
Conclusion: Over the past two decades, VHD trials have expanded in size and geographic scope, with increased enrollment of older adults, particularly in percutaneous and industry-sponsored studies. However, sex disparities and demographic variability persist. Future trials should prioritize inclusive designs to ensure equitable, generalizable evidence for evolving valve interventions.
More abstracts on this topic:
A Randomized Clinical Trial for Asymptomatic Elevated Blood Pressure in Patients Discharged from Emergency Department
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A Comparative Analysis of Social Demographic and Clinical Factors for Screening for Peripheral Artery Disease in Adult Patients from Primary Care ClinicsLane Rashon, Jackson Pasha, Anokwuru Ferdinand, Dillard Naomi, Nerlekar Ridhima