Final ID: MP2248
Sodium–Glucose Cotransporter 2 Inhibitors are Cost-Effective and Dominate Glucagon-Like Peptide-1 Receptor Agonists for Patients with Type 2 Diabetes and High Cardiorenal Risk in Canada
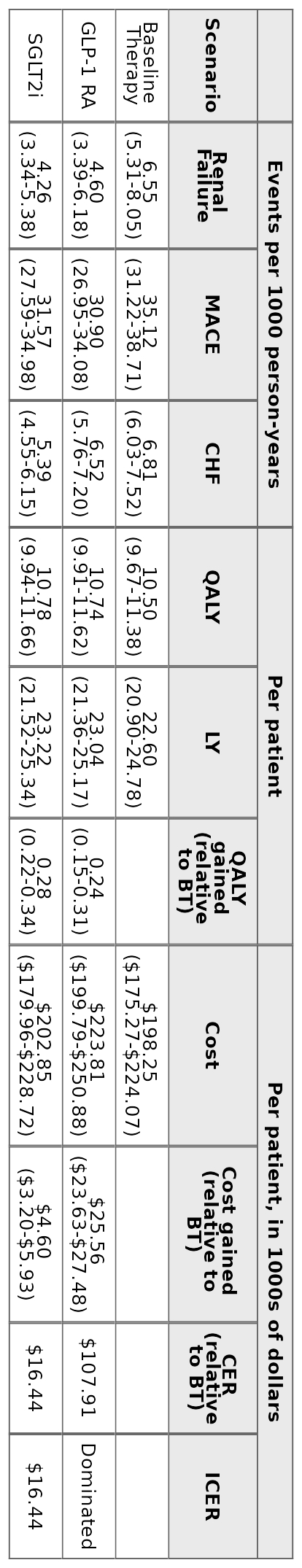
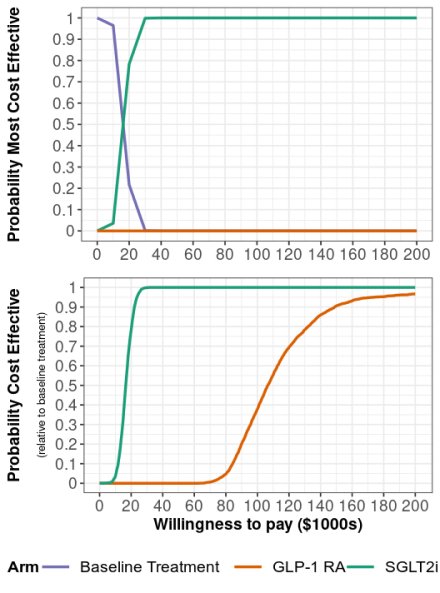
Abstract Body (Do not enter title and authors here): Background: The Canadian Cardiovascular Society recommends that patients with type 2 diabetes (T2D) and cardiovascular disease, renal disease, or multiple cardiovascular risk factors initiate Glucagon-like peptide-1 receptor agonists (GLP-1 RA) or Sodium–glucose cotransporter 2 inhibitors (SGLT2i). Both therapies have demonstrated success reducing deaths, and cardiovascular and renal events for this population but are expensive compared to other T2D treatments. Aims: To assess the cost-effectiveness of SGLT2i and GLP-1 RA in this high-risk population from a Canadian healthcare payer perspective. Methods: We developed a patient-level simulation model to estimate lifetime costs, clinical events, and quality adjusted life years (QALYs) for patients initiating SGLT2i, GLP-1 RA or remaining on “baseline treatment,” i.e., other standard-of-care medications. Simulated patients’ initial laboratory values and demographics were based on 175 Quebec patients referred for GLP-1 RA and/or SGLT2i initiation in 2022–2024. Risk equations and treatment effects were calibrated to endpoints from placebo-controlled cardiovascular outcomes trials. We estimated cost-effectiveness over a patient lifetime horizon using a 3% annual discount rate. Results: Compared to baseline treatment, SGLT2i and GLP-1 RA reduced lifetime rates of cardiovascular and renal events and produced higher net present QALYs (+0.28 SGLT2i; +0.24 GLP-1 RA), but at a higher net present cost (+$4,600 SGLT2i; +$25,560 GLP-1 RA). Per 1000 patient years, the population initiating GLP-1 RA were predicted to have 1.95 fewer renal failure events and 4.22 fewer MACE events compared to baseline treatment. The same population initiating SGLT2i instead were predicted to have 2.29 fewer renal failure events, 3.55 fewer MACE events and 1.42 fewer CHF events compared to baseline treatment. SGLT2i cost $16,440 per QALY gained and were consistently cost-effective in sensitivity analyses at the commonly used threshold of $50,000. GLP-1 RA cost $107,910 per QALY gained and were dominated by SGLT2i in 84% of probabilistic sensitivity analysis iterations. Conclusion: While both treatments improved health outcomes compared to baseline treatment, SGLT2i provided more QALYs at a lower price than GLP-1 RA and are cost-effective at current prices in Canada. The introduction of cheaper generic alternatives and accounting for emerging evidence that these medications impact other clinical outcomes could alter their cost-effectiveness.
More abstracts on this topic:
Adiposomal microRNAs Mediate Vascular Dysfunction in Obesity-Associated Type 2 Diabetes
Mirza Imaduddin, Morsy Mohammed, Levitan Irena, Raj Usha, Mahmoud Abeer
Acetylation of Electron Transfer Flavoprotein Alpha Is a Possible Regulatory Mechanism of Fatty Acid Oxidation in Diabetic HeartsTatekoshi Yuki, Yano Masaki, Hosoda Ryusuke, Saga Yukika, Kuno Atsushi