Final ID: 4342581
Targeting Serotonin 2B Receptor Signaling Attenuates Disease Progression in Hypertrophic Cardiomyopathy
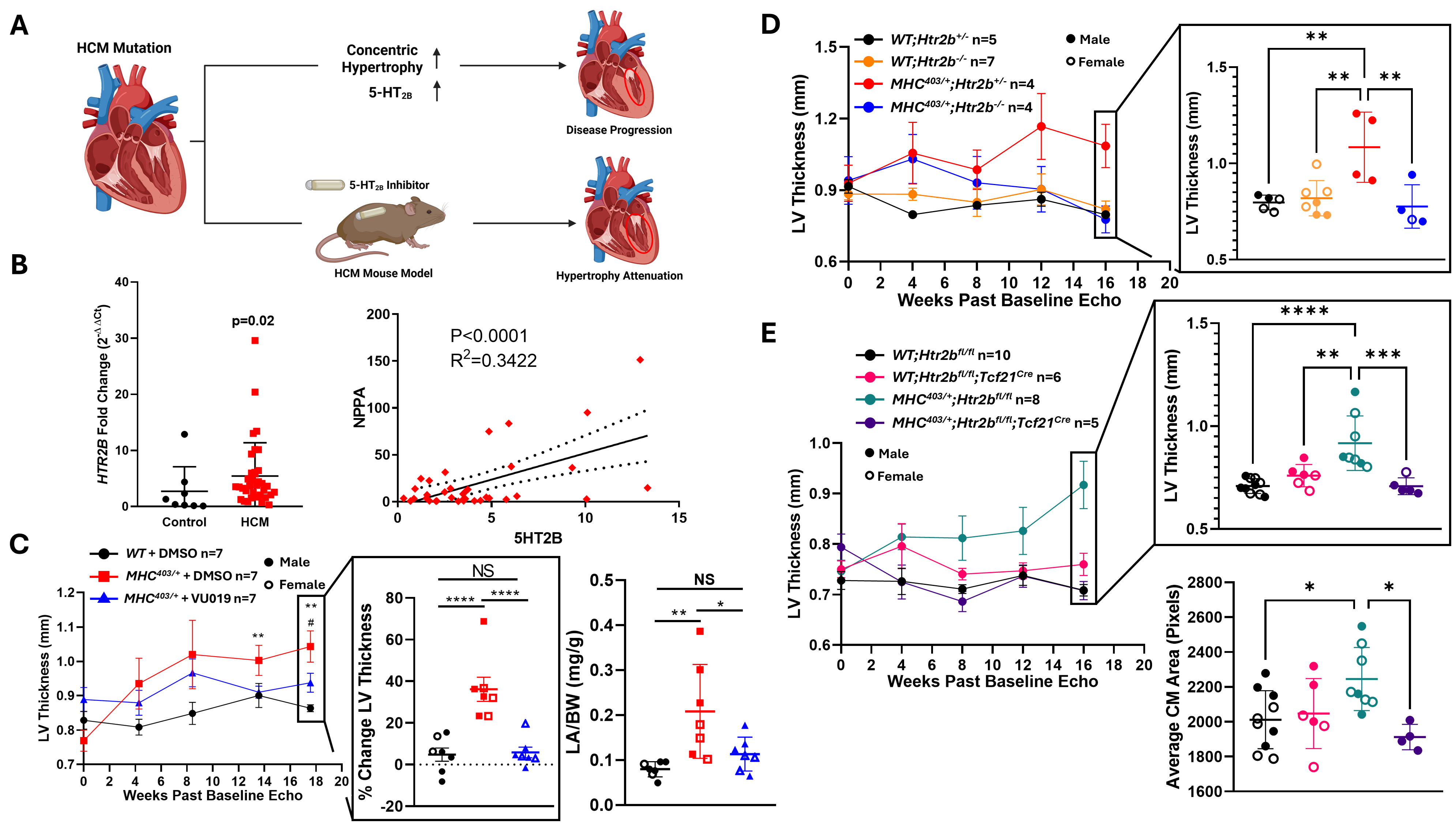
Abstract Body (Do not enter title and authors here): Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease, caused by cardiac myocyte (CM) hypercontractility that induces myocardial hypertrophy and fibrosis. When subjected to pathological stimuli, CM and cardiac fibroblast (CF) activity are mediated by TGF-β1, which has fibrotic and hypertrophic effects on the myocardium and is associated with adverse events in HCM. The serotonin 2B receptor (5-HT2B) is an emerging target for cardiopulmonary diseases, first studied due to the weight-loss medication fen-phen agonizing 5-HT2B. 5-HT2B antagonism alters the fibrotic response in valve disease via abrogation of TGF-β1 signaling. We hypothesize that targeting 5-HT2B will attenuate HCM disease progression by inhibiting the hypertrophic and fibrotic response in CMs and CFs. We obtained human septal myectomy samples from VUMC’s biobank and quantified expression of 5-HT2B and associated biomarkers. We utilized mouse models to investigate inhibition of 5-HT2B in the context of HCM: the α-MHC403/+ (403/+) mouse model that mimics the R403Q human HCM mutation, a global genetic knockout of 5-HT2B, and a fibroblast-specific genetic knockout of 5-HT2B using the Tcf21MCM Cre promoter. We used a novel pharmacological antagonist to inhibit 5-HT2B that is systemically restricted from the brain (Fig1A). Compared to control heart tissue, septal myectomy tissue from HCM patients demonstrate increased 5-HT2B expression and correlation with atrial natriuretic peptide, a marker for ventricular wall stress and hypertrophy (Fig1B). In 403/+ mice given 5-HT2B antagonist, we see left ventricle thickness and left atrial weight is attenuated compared to diseased controls (Fig1C). In both the global and fibroblast-specific 5-HT2B knockout mouse models, we see an attenuation of LV thickness compared to diseased controls (Fig1D and 1E). In addition to attenuating hypertrophy at the level of cardiac structure, we observe that average cardiomyocyte area decreases compared to diseased controls in the fibroblast-specific 5-HT2B knockout mice (Fig1E). This indicates that 5-HT2B signaling in cardiac fibroblasts perpetuates cardiomyocyte hypertrophy. Clearly, pharmacological antagonism of 5-HT2B in HCM has a therapeutic effect in the 403/+ mouse by preventing the progression of myocardial hypertrophy. The specific mechanism by which cardiac fibroblasts and cardiomyocytes co-regulate 5-HT2B-mediated hypertrophy is, to our knowledge, unique to HCM and merits further study.
More abstracts on this topic:
A Cellular Mechanism Mediating Lipomatous Metaplasia In the Infarcted Heart.
Tuleta Izabela, Frangogiannis Nikolaos, Venugopal Harikrishnan, Huang Shuaibo, Humeres Claudio, Hernandez Velasco Silvia, Hanna Anis, Kubota Akihiko, O'leary Kevin, Zheng Deyou
Artificial Intelligence-based screening for Hypertrophic Cardiomyopathy from Single-lead Electrocardiograms: A Multinational Development and Validation StudyCroon Philip, Aminorroaya Arya, Pedroso Aline, Dhingra Lovedeep, Khera Rohan