Final ID: Su2134
The Efficacy and Safety of Plozasiran on Lipid Profile in Dyslipidemic Disorders: A Systematic Review and Meta-Analysis
Abstract Body (Do not enter title and authors here): Background
Dyslipidemias raise the risk of cardiovascular disease and other conditions like fatty liver and pancreatitis. A promising therapeutic target is apolipoprotein C-III (APOC-III), which regulates lipid metabolism. Emerging lipid-lowering therapies, such as Plozasiran, target APOC-III by inhibiting its hepatic production at the mRNA level, presenting a novel approach to lipid regulation. However, the safety and efficacy of plozasiran have yet to be fully established.
Methods
We searched PubMed, Scopus, Web of Science, and Cochrane CENTRAL register of trials for studies comparing plozasiran to placebo in patients with dyslipidemic disorders. The primary outcomes were percentage changes from baseline in triglyceride (TG) and APOC-III levels at the end of the study. Secondary outcomes included changes in other lipid parameters and safety outcomes at the end of the study. A protocol was registered to PROSPERO under registration number [CRD420251026605].
Results
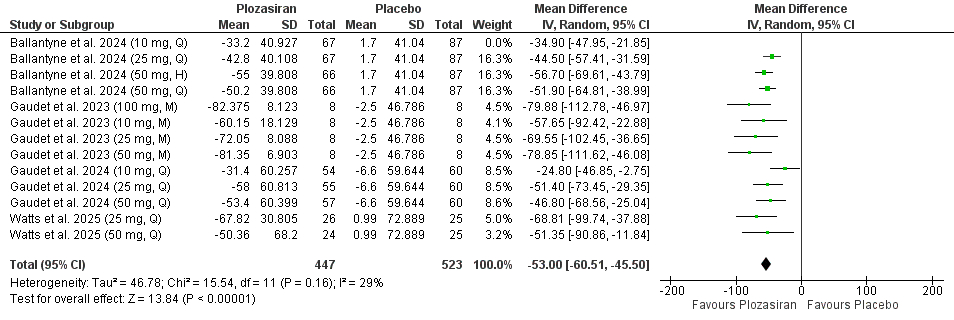
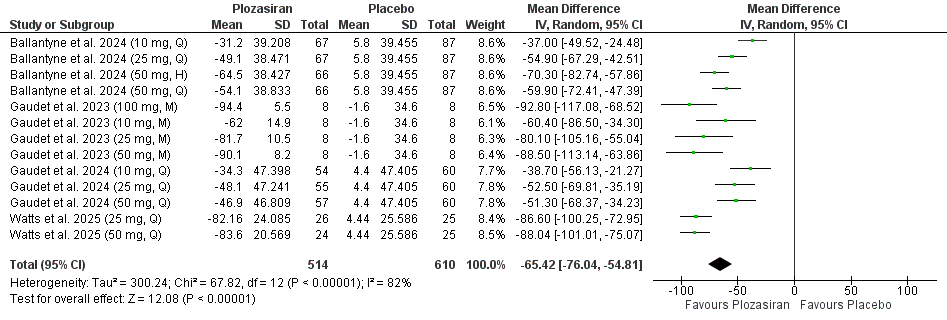
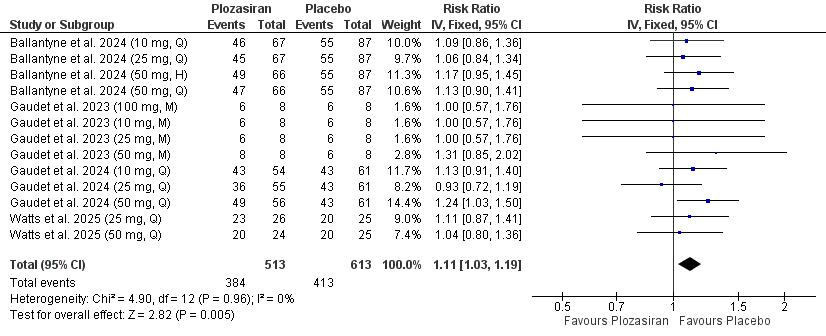
Four studies encompassing 1,514 participants were included in our meta-analysis. Plozasiran significantly improved TGs (MD = -53.00, 95% CI [-60.51, -45.50], P < 0.01), APOC-III (MD = -65.42, 95% CI [-76.04, -54.81], P < 0.01), non-high-density lipoprotein cholesterol (non-HDL-C) (MD = -21.56, 95% CI [-26.69, -16.42], P < 0.01), high-density lipoprotein cholesterol (HDL-C) (MD = 36.73, 95% CI [27.79, 45.67], P < 0.01), and apolipoprotein B (ApoB) (MD = -9.89, 95% CI [-13.19, -6.59], P < 0.01) levels at the end of the study. Subgroup analyses based on dose and dosing frequency revealed consistent findings. Quarterly administration of plozasiran at 10 mg, 25 mg, and 50 mg resulted in significant improvements in TGs, APOC-III, non–HDL-C, and HDL-C at study completion. For ApoB, only the 25 mg and 50 mg quarterly regimens significantly reduced its levels study completion. Regarding safety, patients receiving plozasiran experienced a higher incidence of any adverse events (RR = 1.11, 95% CI [1.03, 1.19], P < 0.01), headache (RR = 1.85, 95% CI [1.14, 3.02], P = 0.01), and mild rises in HbA1C levels (MD = 0.19, 95% CI [0.06, 0.31], P < 0.01).
Conclusion
While Plozasiran shows strong potential as a therapeutic option for severe dyslipidemic conditions, further studies are needed to compare its efficacy and safety with currently available treatments and, more importantly, evaluate its impact on clinical outcomes for implementation in clinical practice.
Dyslipidemias raise the risk of cardiovascular disease and other conditions like fatty liver and pancreatitis. A promising therapeutic target is apolipoprotein C-III (APOC-III), which regulates lipid metabolism. Emerging lipid-lowering therapies, such as Plozasiran, target APOC-III by inhibiting its hepatic production at the mRNA level, presenting a novel approach to lipid regulation. However, the safety and efficacy of plozasiran have yet to be fully established.
Methods
We searched PubMed, Scopus, Web of Science, and Cochrane CENTRAL register of trials for studies comparing plozasiran to placebo in patients with dyslipidemic disorders. The primary outcomes were percentage changes from baseline in triglyceride (TG) and APOC-III levels at the end of the study. Secondary outcomes included changes in other lipid parameters and safety outcomes at the end of the study. A protocol was registered to PROSPERO under registration number [CRD420251026605].
Results
Four studies encompassing 1,514 participants were included in our meta-analysis. Plozasiran significantly improved TGs (MD = -53.00, 95% CI [-60.51, -45.50], P < 0.01), APOC-III (MD = -65.42, 95% CI [-76.04, -54.81], P < 0.01), non-high-density lipoprotein cholesterol (non-HDL-C) (MD = -21.56, 95% CI [-26.69, -16.42], P < 0.01), high-density lipoprotein cholesterol (HDL-C) (MD = 36.73, 95% CI [27.79, 45.67], P < 0.01), and apolipoprotein B (ApoB) (MD = -9.89, 95% CI [-13.19, -6.59], P < 0.01) levels at the end of the study. Subgroup analyses based on dose and dosing frequency revealed consistent findings. Quarterly administration of plozasiran at 10 mg, 25 mg, and 50 mg resulted in significant improvements in TGs, APOC-III, non–HDL-C, and HDL-C at study completion. For ApoB, only the 25 mg and 50 mg quarterly regimens significantly reduced its levels study completion. Regarding safety, patients receiving plozasiran experienced a higher incidence of any adverse events (RR = 1.11, 95% CI [1.03, 1.19], P < 0.01), headache (RR = 1.85, 95% CI [1.14, 3.02], P = 0.01), and mild rises in HbA1C levels (MD = 0.19, 95% CI [0.06, 0.31], P < 0.01).
Conclusion
While Plozasiran shows strong potential as a therapeutic option for severe dyslipidemic conditions, further studies are needed to compare its efficacy and safety with currently available treatments and, more importantly, evaluate its impact on clinical outcomes for implementation in clinical practice.
More abstracts on this topic:
Association of Elevated Lipoprotein(a) Levels with Adverse Outcomes in Patients with Stable Angina Undergoing Stent-less PCI with Paclitaxel-coated Balloon
Takahashi Tomonori, Yamaguchi Koji, Yagi Shusuke, Yamada Hirotsugu, Soeki Takeshi, Sata Masataka, Wakatsuki Tetsuzo, Saijo Yoshihiro, Kawabata Yutaka, Ueno Rie, Kadota Muneyuki, Hara Tomoya, Matsuura Tomomi, Ise Takayuki
A Loss-of-Function Missense Variant in ANGPTL3 Exerts Protective Effects Against Kidney Disease RiskZhang David, Ritchie Marylyn, Rader Daniel, Cuchel Marina