Final ID: MP1579
Incidence and predictors of Monomorphic Ventricular Tachycardia in Three Major Subcutaneous ICD Trials
Abstract Body (Do not enter title and authors here): Background
The subcutaneous ICD (S-ICD) is currently contraindicated for patients (pts) who have monomorphic ventricular tachycardia (MVT) that can be terminated by anti-tachycardia pacing (ATP). Discriminating S-ICD pts who subsequently present with MVT can help identify those who would benefit from a transvenous ICD or upgrade to an investigational, modular leadless pacemaker/S-ICD system to deliver anti-tachycardia pacing.
Objective
Identification of burden and determinants of MVT in S-ICD recipients.
Methods
Pooled data from 3 S-ICD studies: EFFORTLESS (N=994), S-ICD PAS (N=1643), and UNTOUCHED (N=1116) were analyzed. All MVT episodes of all cycle lengths (treated with ≥1 shock and untreated: self-terminated or rate
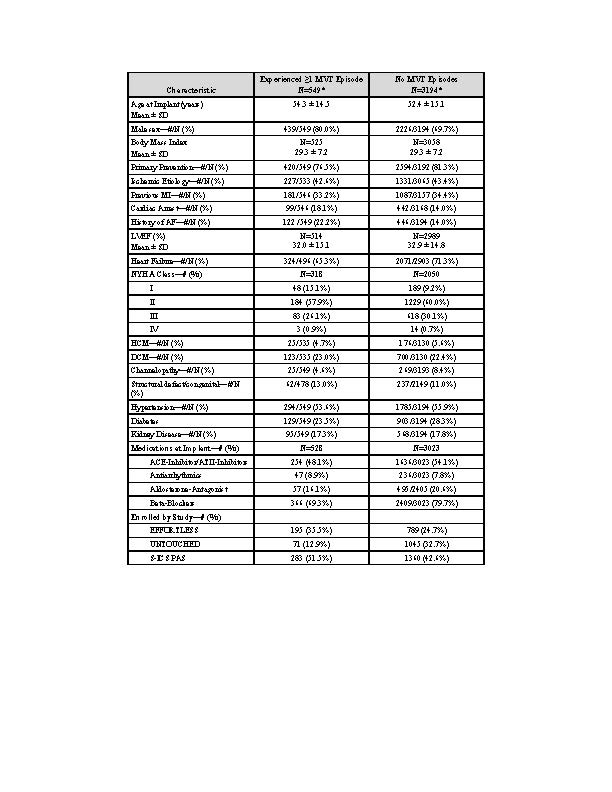
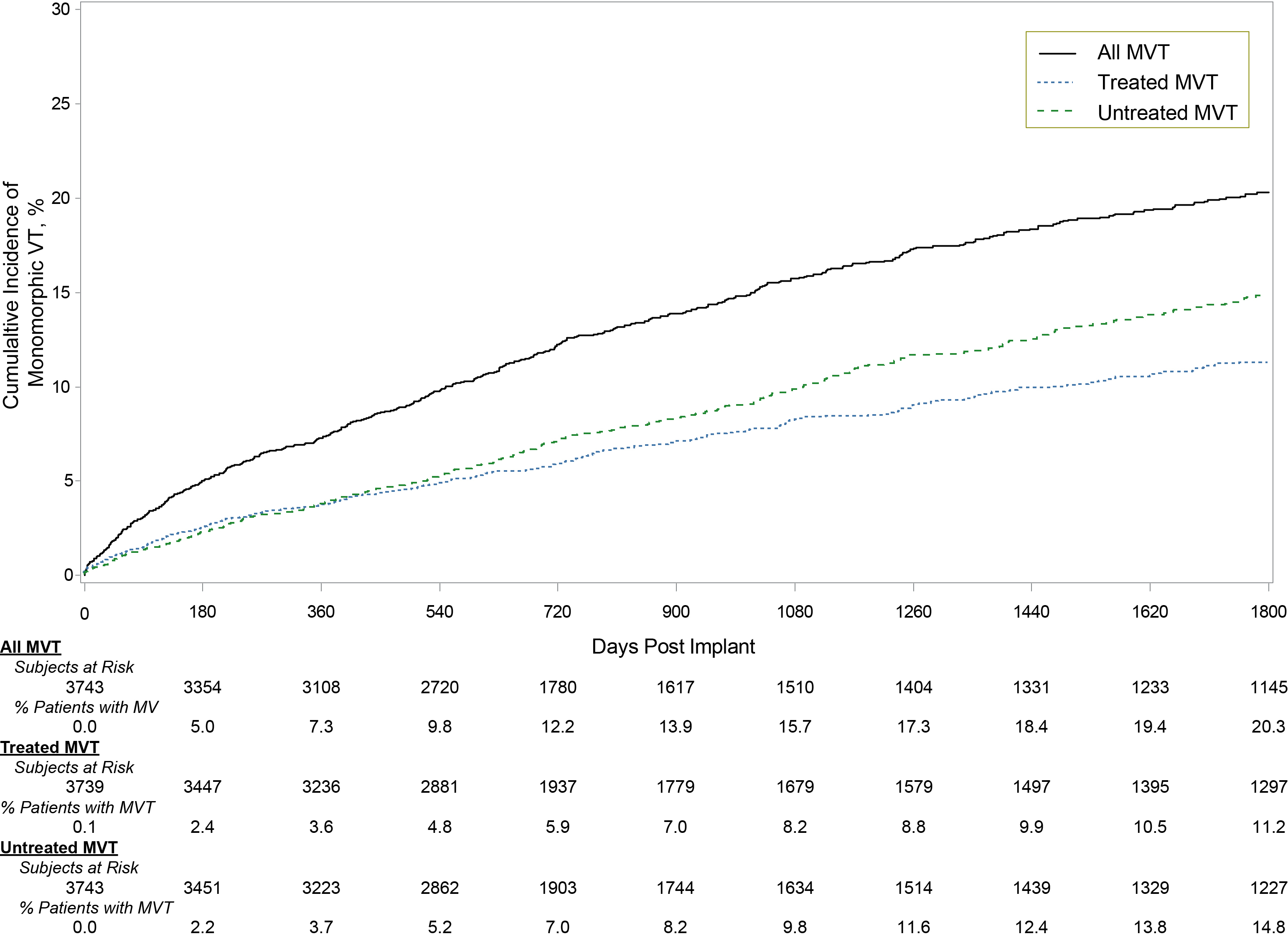
A total of 3753 pts was followed for 3.6 years (median). Of these, 549 (14.6%) experienced >1 MVT episode (table). Age was 54±15 years, 80% male, 77% primary prevention, and EF 32±15%. Of these 549 pts, 373 pts experienced ≥2 episodes, with median time between 1st and 2nd episode 102 (interquartile range 6,391) days. The 5-year rate of experiencing MVT was 20% with shock therapy in 11% (fig1). A total of 298 pts (7.9%) experienced ≥1 shock in 804 episodes, 7% pts had ≥1 shocks for fast MVT (>200bpm), and 52 pts (1.4%) experienced 67 storms (≥3 episodes/24 hours). S-ICD extraction due to the need for ATP pacing was reported for only 10 (0.3%) patients
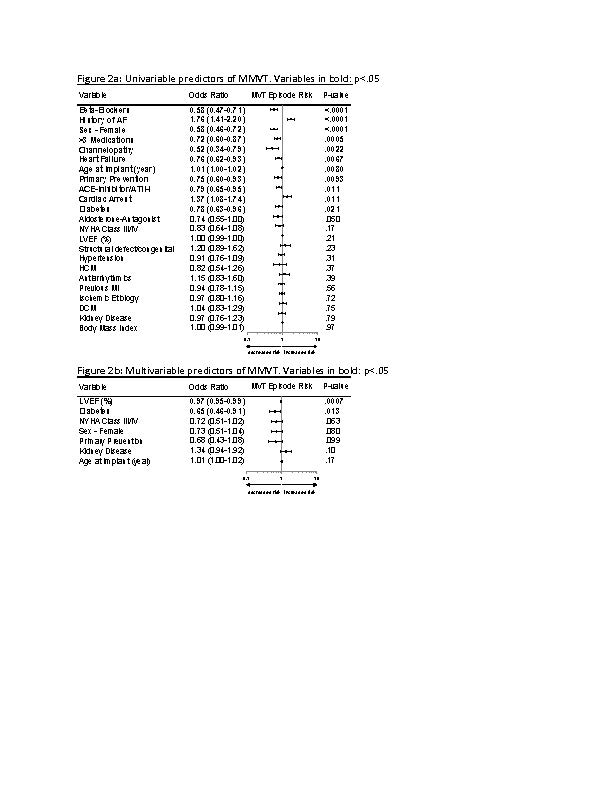
While several baseline variables were identified as a risk of MVT, the multivariable predictor model indicated that pts who experienced MVT were more likely to have a lower ejection fraction and less likely to have diabetes (fig2). Risk of MVT across EF quartiles differed by ≤4.1% with EF 30-35% having the most events and presence of diabetes differed by ≤2.1%, respectively, across all time points.
Conclusions
Over 5 years, 11% of S-ICD patients from EFFORTLESS, S-ICD PAS, and UNTOUCHED studies experienced shock therapy for MVTs, and 20% of patients had MVT (treated and untreated). Conversion rate to TV-ICD for ATP was low. Surprisingly, many of the expected risk factors for MVT were not predictive of MVT, which may be in part due to proper exclusion of patients with a MVT in S-ICD studies. Further detailed analysis of univariable predictor and MVT features could help inform device prescription.
The subcutaneous ICD (S-ICD) is currently contraindicated for patients (pts) who have monomorphic ventricular tachycardia (MVT) that can be terminated by anti-tachycardia pacing (ATP). Discriminating S-ICD pts who subsequently present with MVT can help identify those who would benefit from a transvenous ICD or upgrade to an investigational, modular leadless pacemaker/S-ICD system to deliver anti-tachycardia pacing.
Objective
Identification of burden and determinants of MVT in S-ICD recipients.
Methods
Pooled data from 3 S-ICD studies: EFFORTLESS (N=994), S-ICD PAS (N=1643), and UNTOUCHED (N=1116) were analyzed. All MVT episodes of all cycle lengths (treated with ≥1 shock and untreated: self-terminated or rate
A total of 3753 pts was followed for 3.6 years (median). Of these, 549 (14.6%) experienced >1 MVT episode (table). Age was 54±15 years, 80% male, 77% primary prevention, and EF 32±15%. Of these 549 pts, 373 pts experienced ≥2 episodes, with median time between 1st and 2nd episode 102 (interquartile range 6,391) days. The 5-year rate of experiencing MVT was 20% with shock therapy in 11% (fig1). A total of 298 pts (7.9%) experienced ≥1 shock in 804 episodes, 7% pts had ≥1 shocks for fast MVT (>200bpm), and 52 pts (1.4%) experienced 67 storms (≥3 episodes/24 hours). S-ICD extraction due to the need for ATP pacing was reported for only 10 (0.3%) patients
While several baseline variables were identified as a risk of MVT, the multivariable predictor model indicated that pts who experienced MVT were more likely to have a lower ejection fraction and less likely to have diabetes (fig2). Risk of MVT across EF quartiles differed by ≤4.1% with EF 30-35% having the most events and presence of diabetes differed by ≤2.1%, respectively, across all time points.
Conclusions
Over 5 years, 11% of S-ICD patients from EFFORTLESS, S-ICD PAS, and UNTOUCHED studies experienced shock therapy for MVTs, and 20% of patients had MVT (treated and untreated). Conversion rate to TV-ICD for ATP was low. Surprisingly, many of the expected risk factors for MVT were not predictive of MVT, which may be in part due to proper exclusion of patients with a MVT in S-ICD studies. Further detailed analysis of univariable predictor and MVT features could help inform device prescription.
More abstracts on this topic:
A Systematic Review and Meta-Analysis on the Anesthetic Approaches for Left Atrial Appendage Occlusion: Comparing Conscious Sedation and General Anesthesia
Zaidi Syed Rafay, Ajmal Umna, Rauf Zainab, Maaz Muhammad, Gulzar Sara, Burki Shahid, Nazir Abubakar, Mirza Azka, Hassan Ahmad, Amir Maaz, Jahangir Muhammad Asad, Rasul Minahil, Raza Muhammad, Malik Mohammad
A Phase 2a randomized controlled trial of once-daily versus twice-daily remote ischemic conditioning in vascular cognitive impairment (TRIC-VCI)Ganesh Aravind, Mccreary Cheryl, Sahlas Demetrios, Sharma Mukul, Swartz Richard, Smith Eric, Barber Philip, Black Sandra, Corbett Dale, Field Thalia, Frayne Richard, Hachinski Vladimir, Ismail Zahinoor, Mai Lauren