Final ID: MP1118
Tok Protease Activity Regulates Cardiac Morphogenesis via Slit Fragmentation
Abstract Body (Do not enter title and authors here): Congenital heart defects (CHDs) affect nearly 1% of live births in the United States, yet the developmental mechanisms that guide early heart formation remain poorly understood. Mutations in the evolutionarily conserved metalloprotease Tll1 (Tolloid-like protein 1) lead to atrial-septal defects in vertebrates, with loss-of-function variants representing a common cause of ASD6. However, its cardiac role and molecular targets are not fully defined. To investigate further, we examined the invertebrate homologue Tok (Tolloid-related) in Drosophila melanogaster, a model organism with a simple heart tube and conserved genetic pathways.
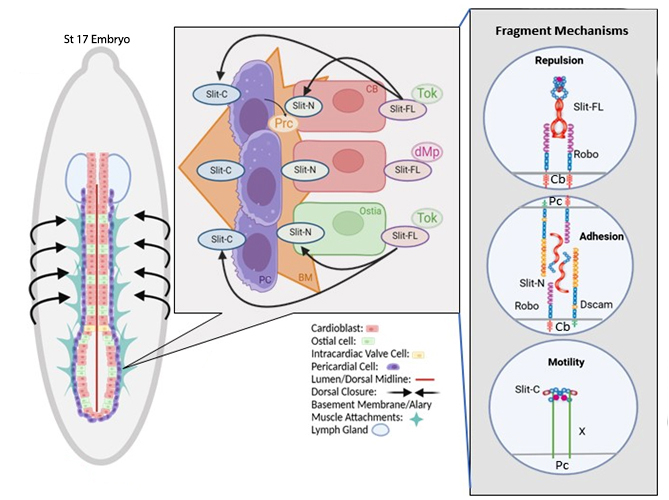
We hypothesize that Tok regulates heart development by cleaving the axon guidance protein, Slit, into functional fragments with distinct roles. To test this, we analyzed tok null mutants and generated new CRISPR-engineered slit alleles: Slit-Uncleavable (slitUC) and a fragment-specific line expressing only the N-terminal (slitN). These genetic tools allowed us to dissect the contributions of Slit fragments to cardiac adhesion and migration.
Our data show that Tok is essential for generating functionally unique Slit fragments with diverse functions in heart development. Slit-N promotes cell-cell adhesion by heterodimerizing with Dscam1 and Robo1 receptors, acting as an adhesive cue between cardiac cells. In the presence of increased Slit-N, adhesion becomes dysregulated, resulting in a “stacked” phenotype and abnormal heart tube structure. Tok loss causes heart defects that mirror mammalian CHDs, including cell depletion, lumen gaps, abnormal rhythm, and structural discontinuities. Remarkably, both Slit-N and Slit-C fragments are capable of rescuing migration and adhesion defects in tok mutants, suggesting that Tok-dependent cleavage is necessary to unlock distinct and complementary functions of Slit.
Preliminary evidence further indicates that Slit fragments may interact with extracellular matrix proteins Pericardin (collagen-IV) and Multiplexin (collagen-XV), implicating Tok in the coordination of both cell-cell and cell-matrix interactions during cardiac development.
Our findings establish Tok as a proteolytic regulator of heart morphogenesis, highlighting distinct roles for the Slit fragments. This work provides new insight into conserved mechanisms that may underlie valve formation and congenital heart defects in humans.
We hypothesize that Tok regulates heart development by cleaving the axon guidance protein, Slit, into functional fragments with distinct roles. To test this, we analyzed tok null mutants and generated new CRISPR-engineered slit alleles: Slit-Uncleavable (slitUC) and a fragment-specific line expressing only the N-terminal (slitN). These genetic tools allowed us to dissect the contributions of Slit fragments to cardiac adhesion and migration.
Our data show that Tok is essential for generating functionally unique Slit fragments with diverse functions in heart development. Slit-N promotes cell-cell adhesion by heterodimerizing with Dscam1 and Robo1 receptors, acting as an adhesive cue between cardiac cells. In the presence of increased Slit-N, adhesion becomes dysregulated, resulting in a “stacked” phenotype and abnormal heart tube structure. Tok loss causes heart defects that mirror mammalian CHDs, including cell depletion, lumen gaps, abnormal rhythm, and structural discontinuities. Remarkably, both Slit-N and Slit-C fragments are capable of rescuing migration and adhesion defects in tok mutants, suggesting that Tok-dependent cleavage is necessary to unlock distinct and complementary functions of Slit.
Preliminary evidence further indicates that Slit fragments may interact with extracellular matrix proteins Pericardin (collagen-IV) and Multiplexin (collagen-XV), implicating Tok in the coordination of both cell-cell and cell-matrix interactions during cardiac development.
Our findings establish Tok as a proteolytic regulator of heart morphogenesis, highlighting distinct roles for the Slit fragments. This work provides new insight into conserved mechanisms that may underlie valve formation and congenital heart defects in humans.
More abstracts on this topic:
Atlas of Distal Nephron Mineralocorticoid Receptor-Dependent Transcriptome Reveals Novel Aldosterone Actions
Welling Paul, Jung Hyun Jun, Su Xiao-tong, Kim Boyoung, Alqusairi Lama, Fenton Robert, Ellison David
A Non-odorant Olfactory Receptor Ligand Depolymerizes the Platelet Actin Cytoskeleton to Prevent ThrombosisAggarwal Anu, Godwin Matthew, Ali Mariya, Jennings Courtney, Rajasekar Bhairavi, Scalise Alliefair, Stauffer Shaun, Mccrae Keith, Mcintyre Thomas, Cameron Scott, Wang Nancy, Josyula Vara Prasad, Yang Moua, Young Shim, Kennedy Quinn, Samuel Reina, Sangwan Naseer, Guntupalli Suman