Final ID: BCVS11
Targeting the TRIM29-PERK signaling axis for treating viral myocarditis
Abstract Body (Do not enter title and authors here): Introduction
Viral myocarditis is an inflammatory disease of the myocardium that significantly contributes to sudden death in children and young adults. The COVID-19 pandemic underscores the critical need to understand the pathogenesis and develop effective treatment strategies for viral myocarditis.
Methods
To investigate the role of the novel E3 ligase TRIM29 in viral myocarditis, we used wild-type and TRIM29 knockout mice infected with coxsackievirus B3 (CVB3) and encephalomyocarditis virus (EMCV), to induce the myocarditis. Additionally, wild-type mice infected with CVB3 were treated with the protein kinase RNA-like endoplasmic reticulum kinase (PERK) inhibitor GSK2656157 or a DMSO control to evaluate potential therapeutic interventions. Mice survival and heart function were monitored using transthoracic echocardiography, and hearts were harvested for histological and immunohistochemical analysis. Techniques including real-time PCR, western blotting, co-immunoprecipitation, enzyme-linked immunoassay, flow cytometry, over-expression, and knockdown were employed to elucidate the pathogenic mechanisms by which TRIM29 regulates the endoplasmic reticulum stress response after viral infection.
Results
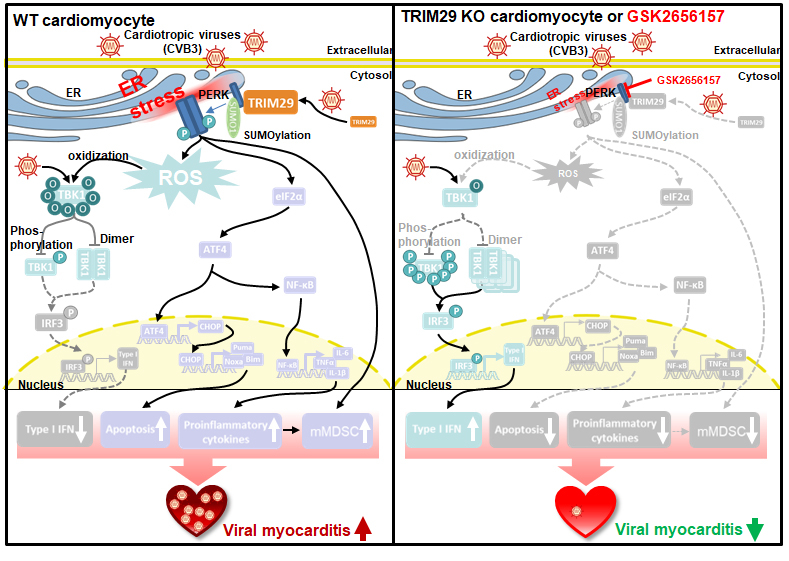
We found that TRIM29 was highly induced by cardiotropic viruses, including CVB3 andEMCV, and promoted PERK-mediated endoplasmic reticulum (ER) stress, apoptosis, and reactive oxygen species (ROS) responses, which restricted viral replication in cardiomyocytes in vitro. TRIM29 deficiency protected mice from viral myocarditis by enhancing cardiac antiviral functions and reducing PERK-mediated inflammation and immunosuppressive cells in vivo. Mechanistically, TRIM29 interacted with PERK to catalyze the SUMOylation of PERK, maintaining its stability and thereby promoting PERK-mediated signaling pathways. Furthermore, we demonstrated that the PERK inhibitor GSK2656157 mitigated viral myocarditis by disrupting the TRIM29-PERK interaction, which improved cardiac function, enhanced cardiac antiviral responses, and reduced inflammation and immunosuppressive cells in vivo.
Conclusions
Our findings provide the first evidence that the TRIM29-PERK signaling axis can be targeted for the treatment of viral myocarditis. This suggests that targeting the TRIM29-PERK axis could effectively mitigate viral myocarditis and associated cardiovascular diseases.
Viral myocarditis is an inflammatory disease of the myocardium that significantly contributes to sudden death in children and young adults. The COVID-19 pandemic underscores the critical need to understand the pathogenesis and develop effective treatment strategies for viral myocarditis.
Methods
To investigate the role of the novel E3 ligase TRIM29 in viral myocarditis, we used wild-type and TRIM29 knockout mice infected with coxsackievirus B3 (CVB3) and encephalomyocarditis virus (EMCV), to induce the myocarditis. Additionally, wild-type mice infected with CVB3 were treated with the protein kinase RNA-like endoplasmic reticulum kinase (PERK) inhibitor GSK2656157 or a DMSO control to evaluate potential therapeutic interventions. Mice survival and heart function were monitored using transthoracic echocardiography, and hearts were harvested for histological and immunohistochemical analysis. Techniques including real-time PCR, western blotting, co-immunoprecipitation, enzyme-linked immunoassay, flow cytometry, over-expression, and knockdown were employed to elucidate the pathogenic mechanisms by which TRIM29 regulates the endoplasmic reticulum stress response after viral infection.
Results
We found that TRIM29 was highly induced by cardiotropic viruses, including CVB3 andEMCV, and promoted PERK-mediated endoplasmic reticulum (ER) stress, apoptosis, and reactive oxygen species (ROS) responses, which restricted viral replication in cardiomyocytes in vitro. TRIM29 deficiency protected mice from viral myocarditis by enhancing cardiac antiviral functions and reducing PERK-mediated inflammation and immunosuppressive cells in vivo. Mechanistically, TRIM29 interacted with PERK to catalyze the SUMOylation of PERK, maintaining its stability and thereby promoting PERK-mediated signaling pathways. Furthermore, we demonstrated that the PERK inhibitor GSK2656157 mitigated viral myocarditis by disrupting the TRIM29-PERK interaction, which improved cardiac function, enhanced cardiac antiviral responses, and reduced inflammation and immunosuppressive cells in vivo.
Conclusions
Our findings provide the first evidence that the TRIM29-PERK signaling axis can be targeted for the treatment of viral myocarditis. This suggests that targeting the TRIM29-PERK axis could effectively mitigate viral myocarditis and associated cardiovascular diseases.
More abstracts on this topic:
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulin
Dabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey
A Novel Cardioprotective Mechanism in Myocardial Reperfusion Injury: Dual Neutrophil Modulation and ROS/HOCl Scavenging by an Atypical ChemokineZwissler Leon, Bernhagen Juergen, Cabrera-fuentes Hector Alejandro, Hernandez Resendiz Sauri, Yap En Ping, Schindler Lisa, Zhang Zhishen, Dickerhof Nina, Hampton Mark, Liehn Elisa, Hausenloy Derek