Final ID: 4171199
Benefits of Semaglutide on Chronic Kidney Disease Outcomes by Cardiovascular Status or Risk in the FLOW trial
Study Design and Methods: A double-blind, randomized (1:1) phase 3 trial.
Sample Size: N=3533.
Population Studied: FLOW included participants with type 2 diabetes and CKD (estimated glomerular filtration rate [eGFR] 50–75 mL/min/1.73 m2 and urine albumin–creatinine ratio [UACR] >300–<5000 mg/g, or, eGFR 25–<50 mL/min/1.73 m2 and UACR >100–<5000 mg/g). Analyses were based on participant categorization at baseline by prior myocardial infarction (MI), stroke, MI or stroke, peripheral artery disease (PAD), or total CV risk in those without CV disease at baseline (PREVENT score; <20%/≥20%).
Intervention(s): Subcutaneous semaglutide 1.0 mg weekly or placebo.
Power Calculations: Event driven and designed for 90% power to detect a 20% relative risk reduction for the primary kidney outcome. There were no specific power calculations for subgroup analyses, which were pre-specified except for the PREVENT analysis.
Primary Outcome: A composite of kidney failure (eGFR <15 mL/min/1.73m2, dialysis, transplant), ≥50% eGFR decline from baseline, and kidney or CV death.
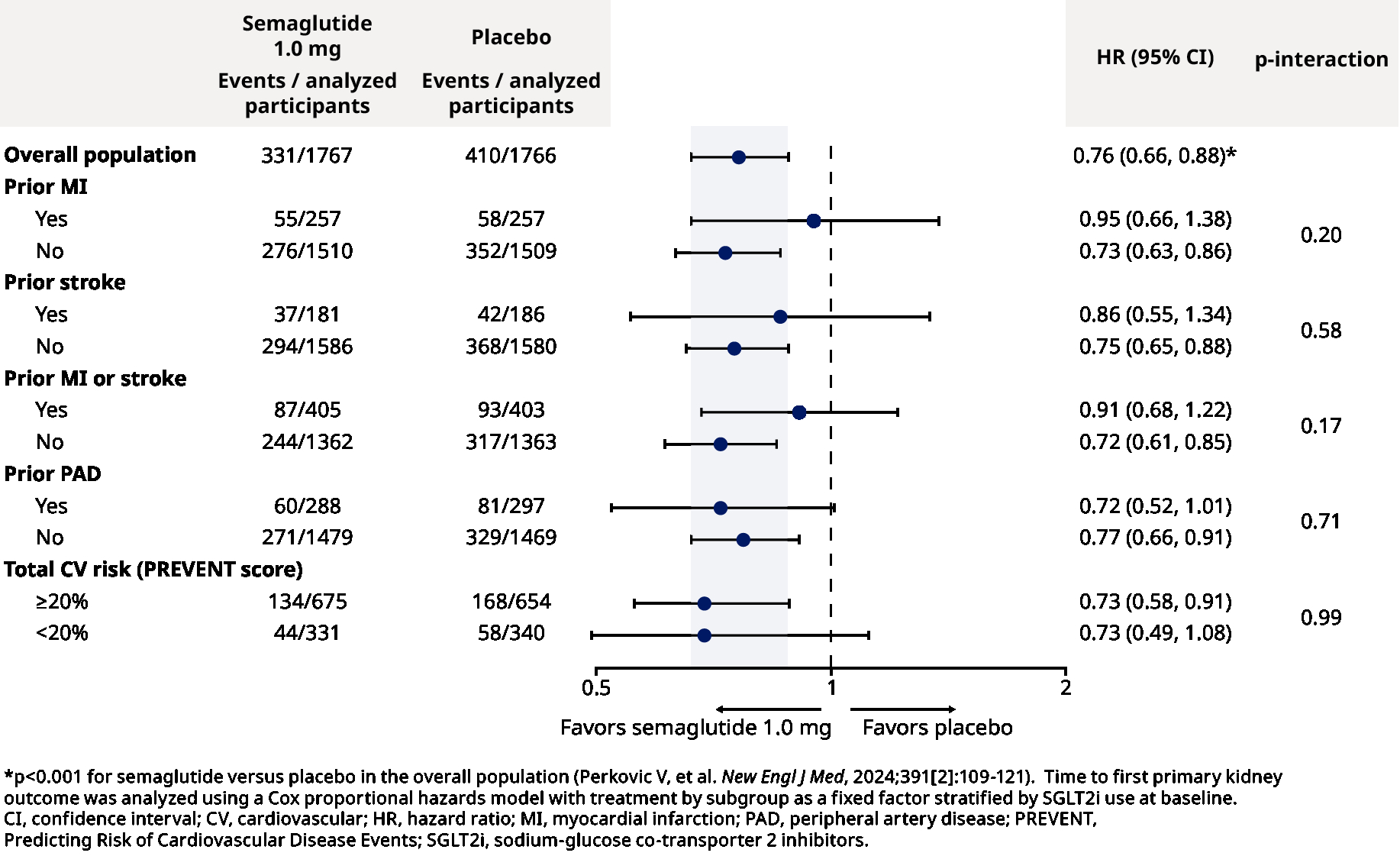
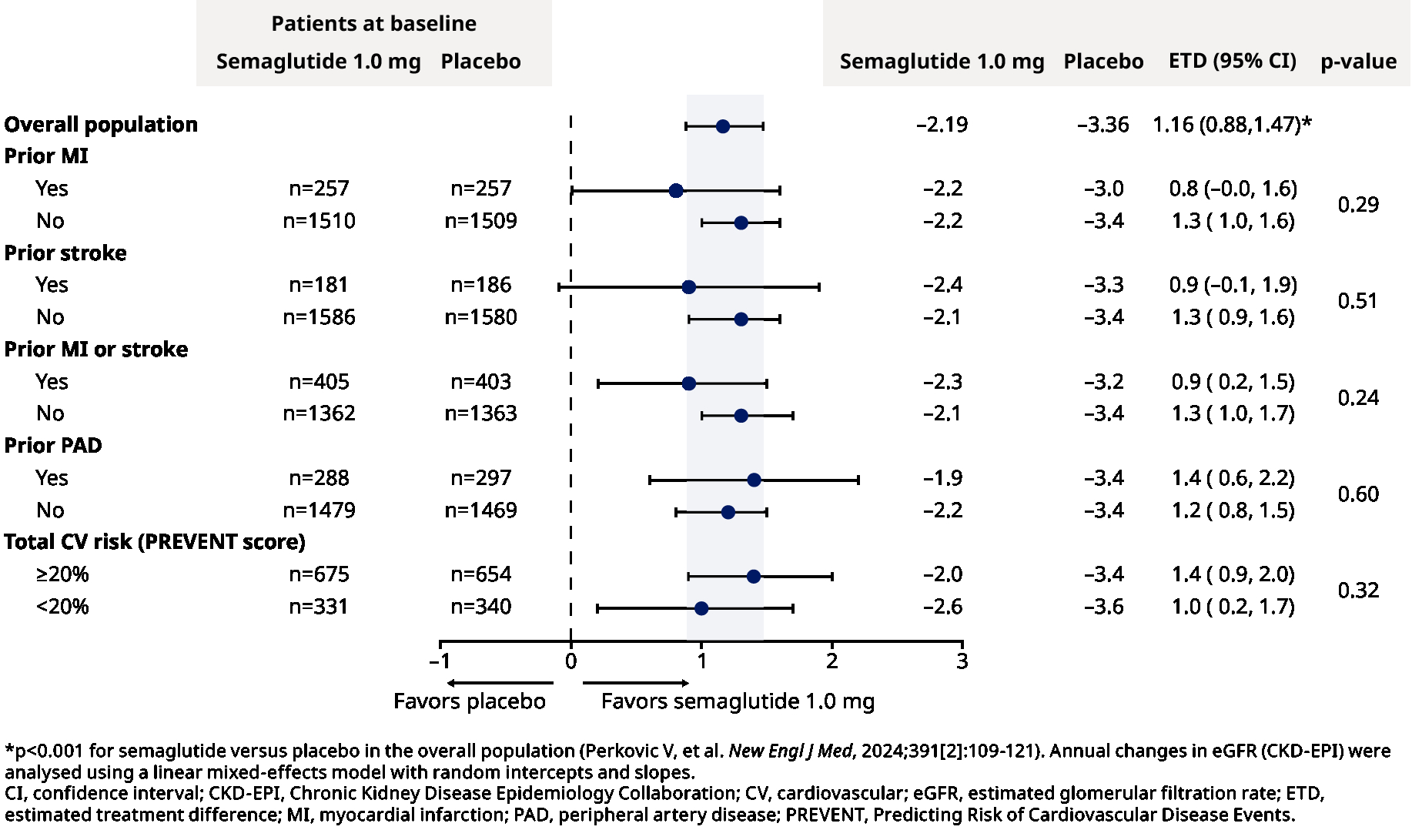
Outcomes: Participants were followed for a median of 3.4 years. Their mean age was 67 years; 30% were women; mean eGFR was 47.0 mL/min/1.73 m2; median UACR was 568 mg/g. Compared to placebo, semaglutide resulted in a 24% relative risk reduction in the primary kidney outcome (hazard ratio 0.76; 95% confidence interval 0.66, 0.88). Effects of semaglutide on the primary kidney outcome (Figure 1) and annual change in eGFR (Figure 2) were consistent across subgroups according to prior MI, stroke, MI or stroke, PAD, or total CV risk at baseline (all p-heterogeneity >0.17).
Conclusions: In the FLOW trial, the benefits of semaglutide on CKD outcomes in participants with type 2 diabetes were similar regardless of baseline CV status or risk.
- Tuttle, Katherine ( University of Washington , Spokane , Washington , United States )
- Mann, Johannes ( German Heart Center , Munich , Germany )
- Perkovic, Vlado ( University of New South Wales , Sidney , New South Wales , Australia )
- Pratley, Richard ( AdventHealth Orlando , Orlando , Florida , United States )
- Ridker, Paul ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Schmieder, Roland ( University Erlangen Nimberg , Erlangen , Germany )
- Shamkhalova, Minara S. ( Endocrinology Research Centre , Moscow , Romania )
- Sreenivasamurthy, L ( Lifecare Hospital and Research Centre , Bangalore, , India )
- Mahaffey, Kenneth ( Stanford University , Stanford , California , United States )
- Bakris, George ( University of Chicago , Chicago , Illinois , United States )
- Bang, Casper ( Novo Nordisk , Copenhagen , Denmark )
- Bax, Willem ( Northwest Clinics , Alkmaar , Netherlands )
- Belmar, Nicolas ( Novo Nordisk , Copenhagen , Denmark )
- Brown, Paul ( Novo Nordisk , Copenhagen , Denmark )
- Cherney, David ( Toronto General Hospital , Toronto , Ontario , Canada )
- Chernin, Gil ( KAPLAN MEDICAL CENTER , Tel Aviv , Israel )
- Lim, Soo Kun ( University Malaya , Kuala Lumpur , Malaysia )
Meeting Info:
Session Info:
Featured Science: Incretin Modulation: Is a New Standard of Care Emerging?
Monday, 11/18/2024 , 01:30PM - 02:45PM
Featured Science
More abstracts on this topic:
Abdollahi Ashkan, Rotter Jerome, Post Wendy, Blumenthal Roger, Bluemke David, Lima Joao Ac, Whelton Seamus, Sani Maryam, Shabani Mahsima, Scarpa Bruna, Blaha Michael, Wu Colin, Ambale-venkatesh Bharath, Budoff Matthew, Strom Jordan
3-Minute Heart Health App: A Feasibility StudyAbdulkarim Iya, Metzger Joseph, Stovitz Steven, Van't Hof Jeremy
More abstracts from these authors:
Tuttle Katherine, Pratley Richard
Don't Forget about the Kidneys!Rangaswami Janani, Cherney David