Final ID: 4169811
Efficacy and Safety of Edoxaban in Anticoagulant Therapy Early after Surgical Bioprosthetic Valve Replacement: ENBALV trial
Abstract Body (Do not enter title and authors here): Background: The incidence of embolic events has been reported to be high early after bioprosthetic valve (BPV) replacement, thus the current guidelines recommend anticoagulant therapy with vitamin K antagonists for 3 to 6 months after operation. However, warfarin has a narrow therapeutic range and interacts with other drugs and food.
Hypothesis and Purpose: Our hypothesis is that the efficacy and safety of edoxaban are comparable to warfarin in patients early after BPV replacement.
Study Design: An investigator-initiated, phase 3, randomized, open-label, multicenter study (jRCT2051210209).
Population Studied: Patients aged 18 to 85 years undergoing BPV replacement at the aortic and/or mitral position.
Intervention: Edoxaban versus warfarin
Methods: Patients were randomized 1:1 to receive either edoxaban (60 or 30mg once daily adjusted by renal function, body weight, and concomitant use of P-glycoprotein inhibitors) or warfarin (dose adjusted under monitoring PT-INR). Administration of edoxaban or warfarin was continued for 12 weeks after surgery.
Sample Size: The sample size was set at 450 to account for 10% dropout.
Power Calculations: The event incidence rates in the edoxaban and warfarin groups were assumed to be 1-3% each. A difference of no more than 2% in the event incidence rate between the edoxaban group and warfarin group was considered a clinically acceptable margin. The probability that the point estimate of the difference in event incidence rates would fall within this threshold was calculated to be approximately 90%.
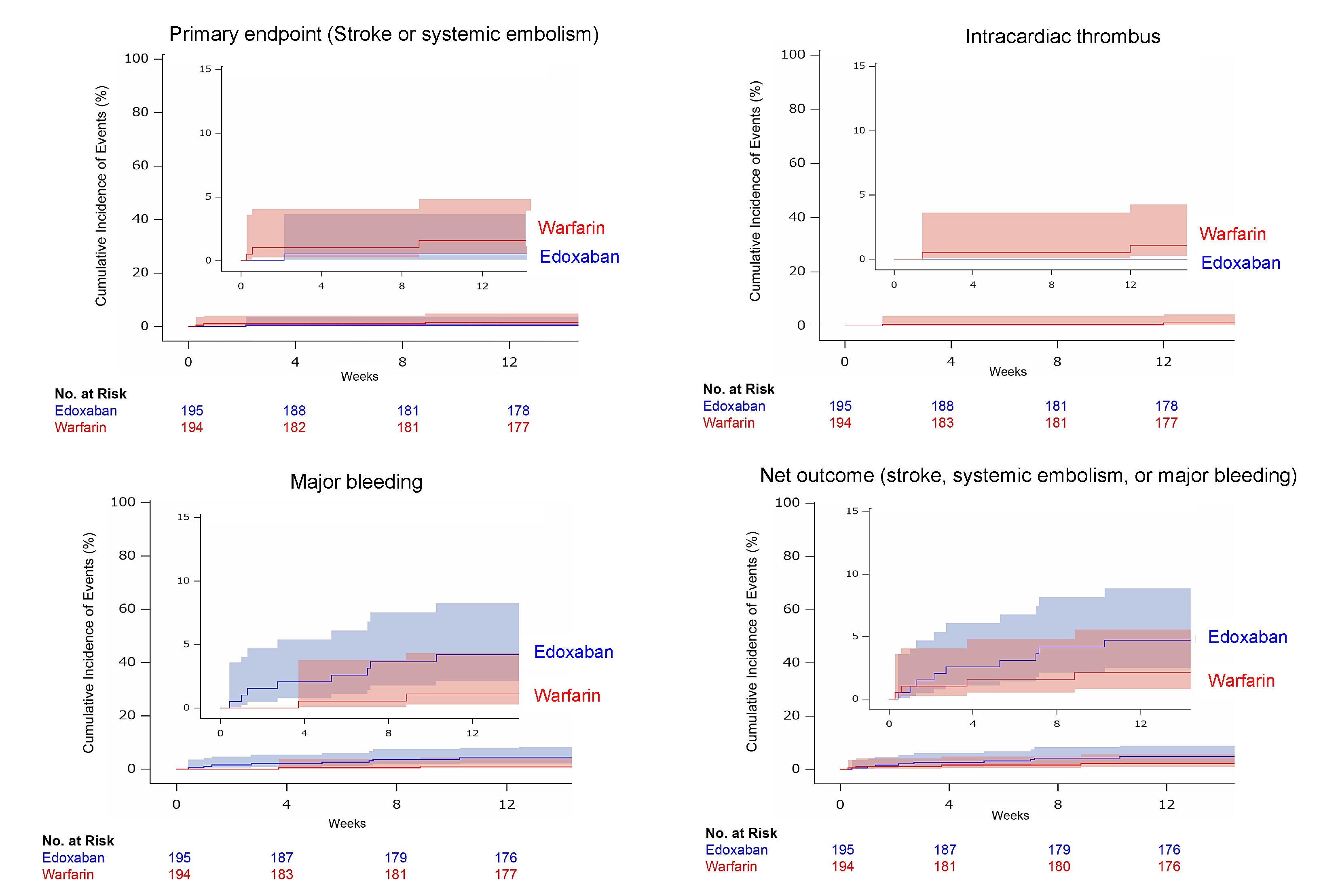
Primary End Points: Stroke or systemic embolism at 12 weeks after surgery.
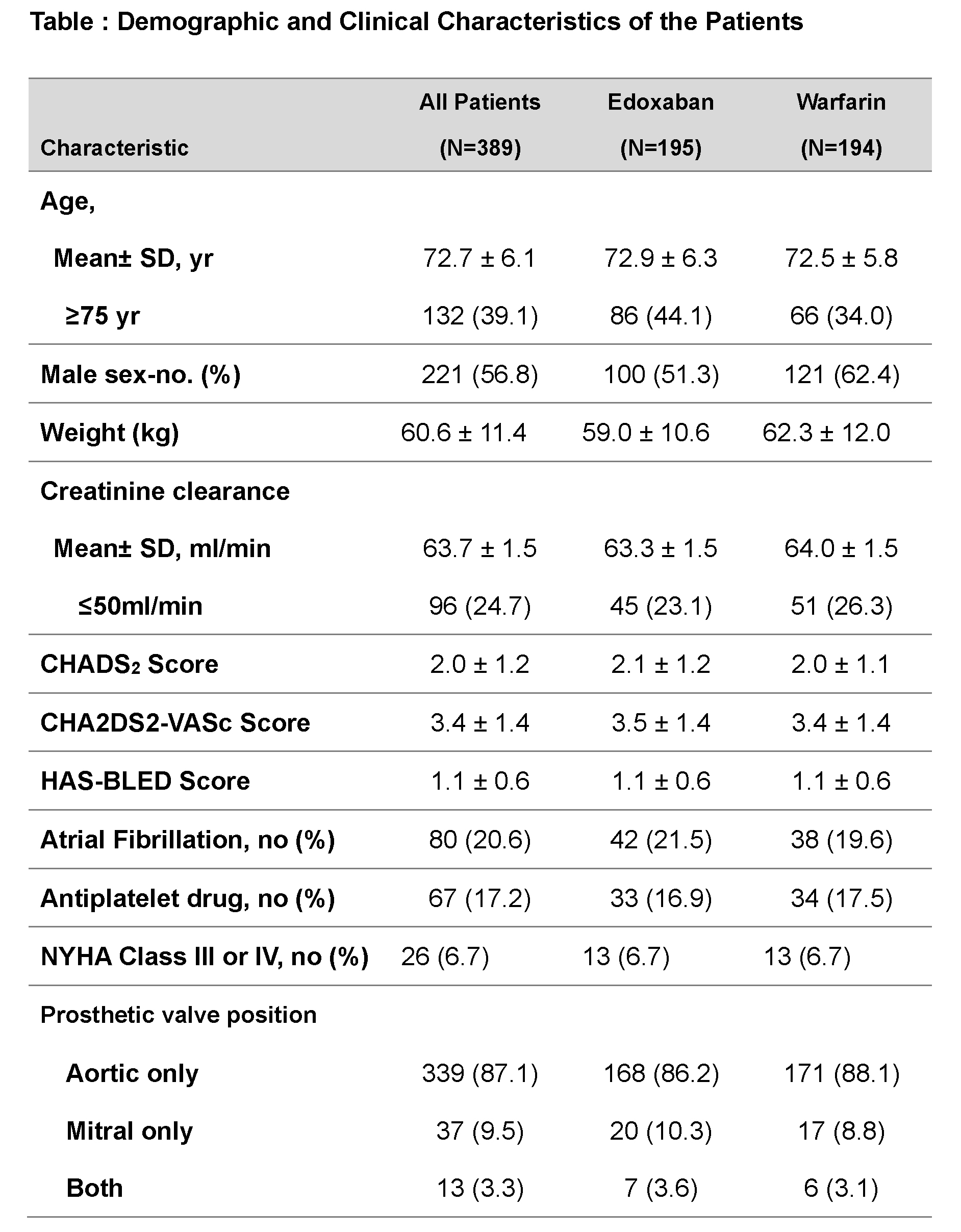
Secondary End Points: Major bleeding, intracardiac thrombus, and a composite of stroke, systemic embolism, or major bleeding.
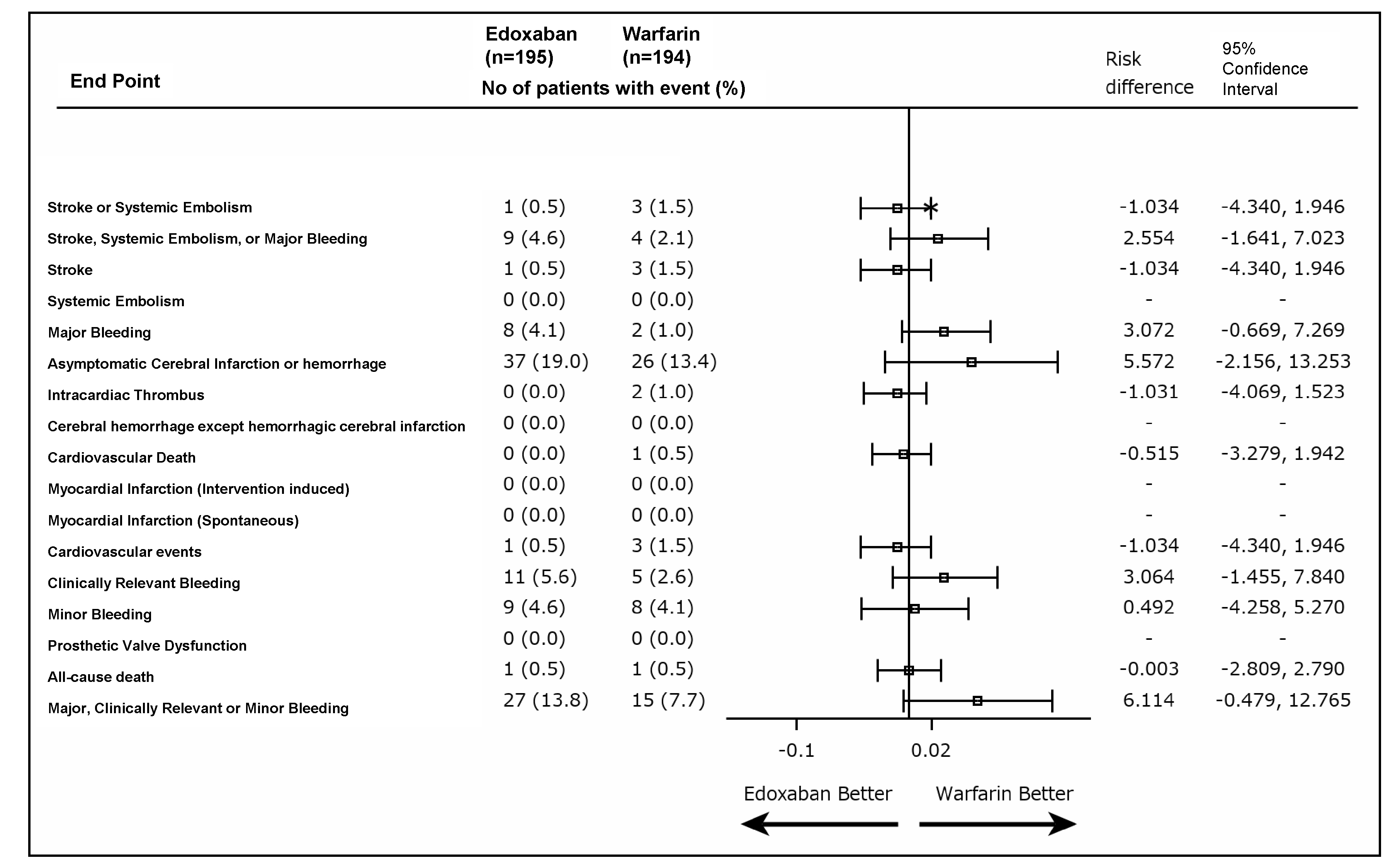
Outcomes: Of 410 enrolled patients, 389 were included in the final analysis (73±6 years, 56.8% male, 79.4% sinus rhythm, edoxaban group: n=195, warfarin group: n=194). The primary outcome occurred in 0.5% (n=1) in the edoxaban group, whereas in 1.5% (n=3) in the warfarin group (risk difference, −1.03, 95%CI, −4.34 to 1.95). The incidence of major bleeding was numerically higher in the edoxaban group (4.1% vs 1.0%, risk difference, 3.07; 95%CI, −0.67 to 7.27), but no fatal bleeding or intracranial hemorrhage was observed, whereas one fatal intracranial hemorrhage occurred in the warfarin group. Our results suggest that edoxaban is a potential alternative anticoagulant therapy early after BPV replacement, including patients with sinus rhythm.
Hypothesis and Purpose: Our hypothesis is that the efficacy and safety of edoxaban are comparable to warfarin in patients early after BPV replacement.
Study Design: An investigator-initiated, phase 3, randomized, open-label, multicenter study (jRCT2051210209).
Population Studied: Patients aged 18 to 85 years undergoing BPV replacement at the aortic and/or mitral position.
Intervention: Edoxaban versus warfarin
Methods: Patients were randomized 1:1 to receive either edoxaban (60 or 30mg once daily adjusted by renal function, body weight, and concomitant use of P-glycoprotein inhibitors) or warfarin (dose adjusted under monitoring PT-INR). Administration of edoxaban or warfarin was continued for 12 weeks after surgery.
Sample Size: The sample size was set at 450 to account for 10% dropout.
Power Calculations: The event incidence rates in the edoxaban and warfarin groups were assumed to be 1-3% each. A difference of no more than 2% in the event incidence rate between the edoxaban group and warfarin group was considered a clinically acceptable margin. The probability that the point estimate of the difference in event incidence rates would fall within this threshold was calculated to be approximately 90%.
Primary End Points: Stroke or systemic embolism at 12 weeks after surgery.
Secondary End Points: Major bleeding, intracardiac thrombus, and a composite of stroke, systemic embolism, or major bleeding.
Outcomes: Of 410 enrolled patients, 389 were included in the final analysis (73±6 years, 56.8% male, 79.4% sinus rhythm, edoxaban group: n=195, warfarin group: n=194). The primary outcome occurred in 0.5% (n=1) in the edoxaban group, whereas in 1.5% (n=3) in the warfarin group (risk difference, −1.03, 95%CI, −4.34 to 1.95). The incidence of major bleeding was numerically higher in the edoxaban group (4.1% vs 1.0%, risk difference, 3.07; 95%CI, −0.67 to 7.27), but no fatal bleeding or intracranial hemorrhage was observed, whereas one fatal intracranial hemorrhage occurred in the warfarin group. Our results suggest that edoxaban is a potential alternative anticoagulant therapy early after BPV replacement, including patients with sinus rhythm.
More abstracts on this topic:
Catastrophic Bioprosthetic Aortic Valve Thrombosis: An Unappreciated Complication of Veno-Arterial Extracorporeal Membrane Oxygenation Support in Cardiogenic Shock
O'hara Patrick, Heid Christopher, Lahsaei Peiman, Farr Maryjane, Garg Sonia, Araj Faris, Truby Lauren, Goral Montana, Kim Han, Malensek Paris, Beaini Hadi, Patel Ravi, Jawaid Anas, Hussain Fizza
A Rare Case of Sequential Impella Mechanical Failures due to Infective Endocarditis VegetationsSawalski Cathryn, Seu Michelle, Darki Amir