Final ID: MDP269
Real world analysis of adverse events with implantation of aveir leadless pacemaker in comparison to micra leadless pacemaker: a food and drug administration MAUDE database study
Abstract Body (Do not enter title and authors here): Background: Leadless pacemaker (LP) is a novel pacemaker offering an innovative approach to bradyarrhythmia treatment. Aveir LP and Micra LP are the two leadless pacing systems available in the United States. Aveir LP was approved by the Food and Drug Administration (FDA) in April 2022. Data regarding the adverse events (AE) following implantation of Aveir LP is scarce, largely limited to single centers, and no real-world comparative analyses were done previously.
Methods: We queried the FDA Manufacturer and User Facility Device Experience (MAUDE) database between April 2022 and December 2023 to assess the safety and AE following implantation of Aveir LP. "AVIER" and "MICRA" were the key terms used to search the MAUDE database. The event types "death" and "injury" were included in our search to capture major clinical events related to the patient. Disproportionality analysis was performed using the reporting odds ratio (ROR) to compare the adverse events of Aveir LP with Micra LP. A signal to noise ratio was considered to be significant if the confidence interval (CI) did not cross the number "one".
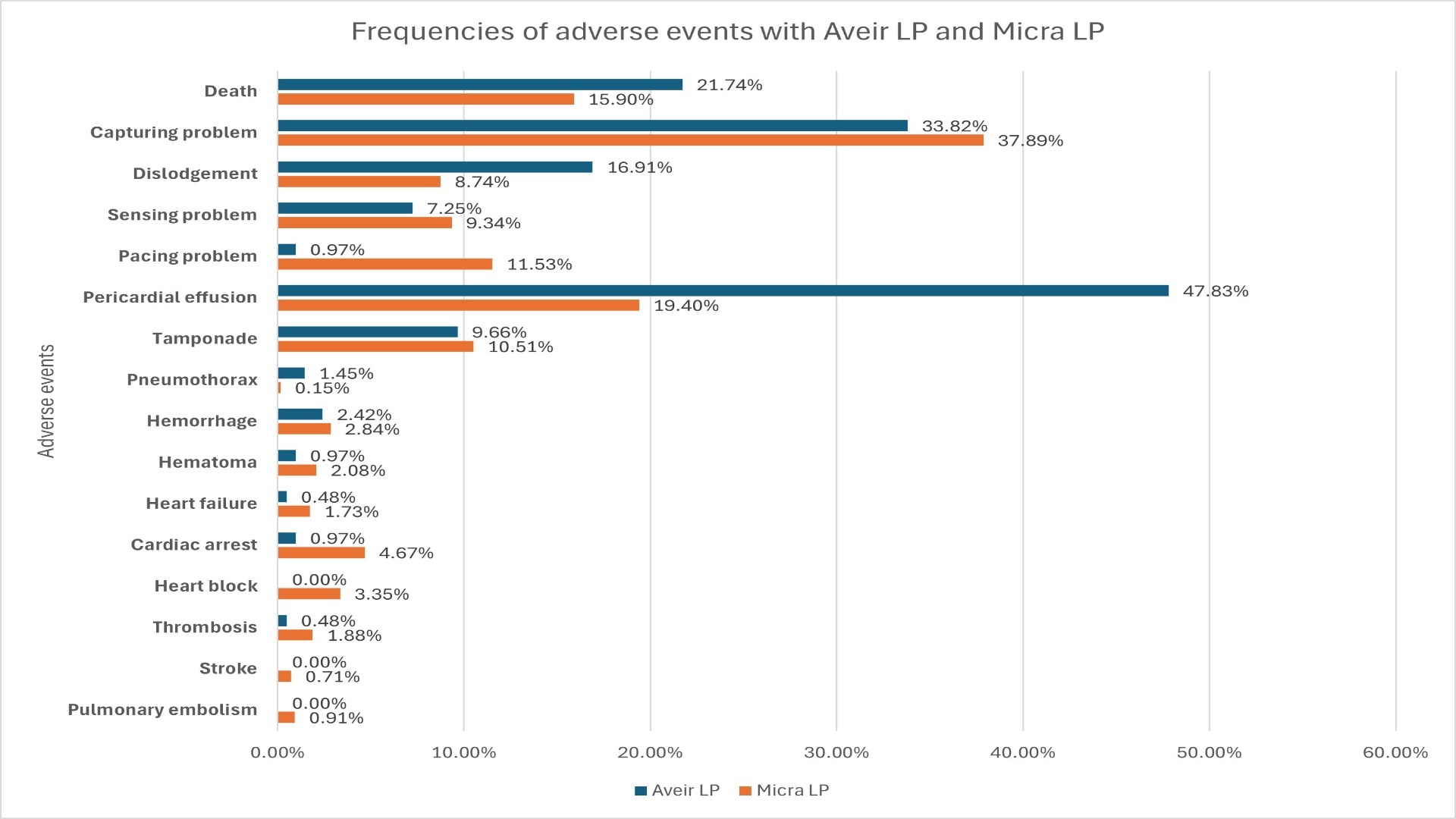
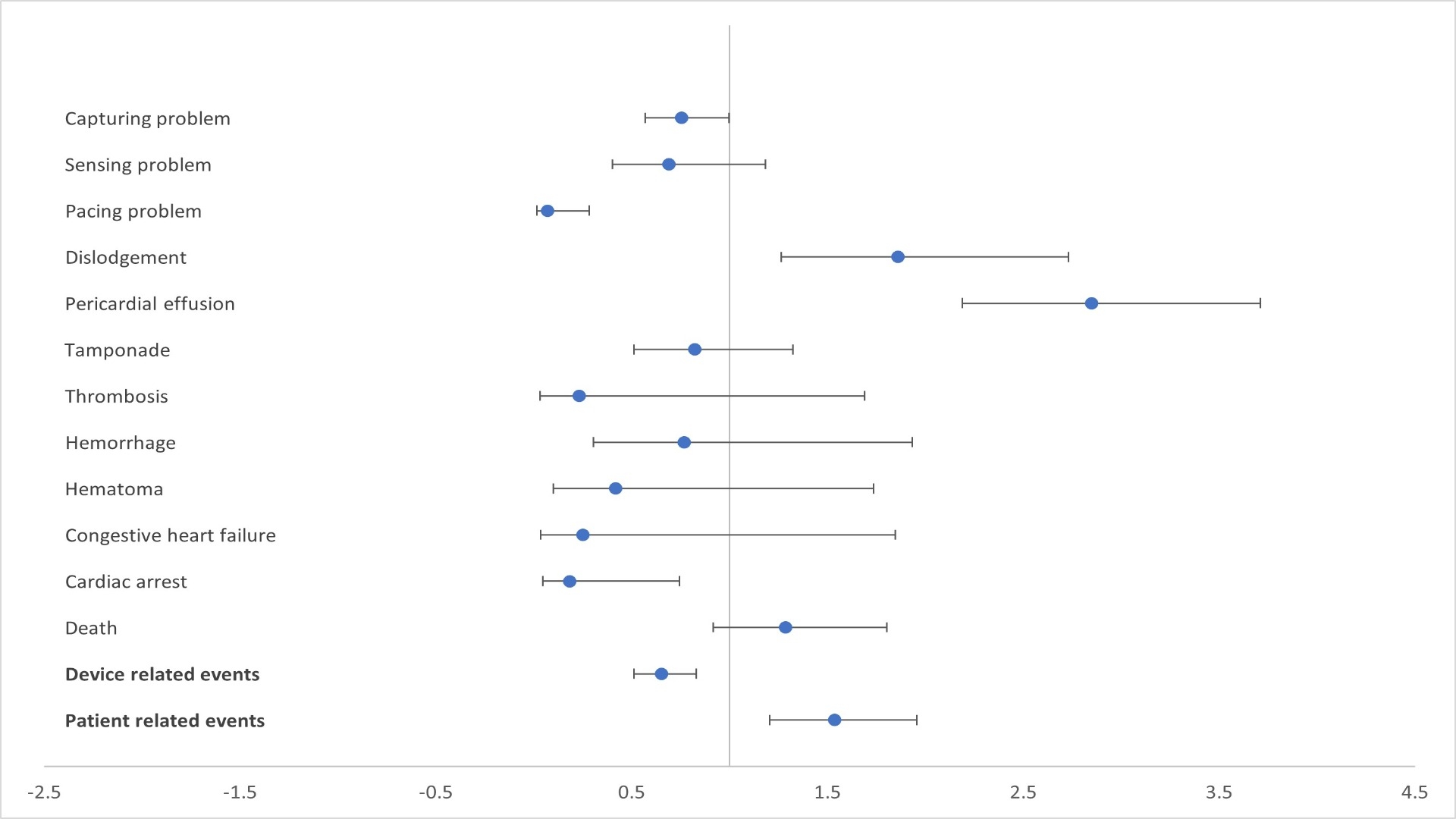
Results: Our search resulted in 207 event reports for Aveir LP and 1969 event reports for Micra LP. Major device related adverse events with Aveir LP were capturing problem (33.8%) followed by dislodgement (16.9%), and sensing problem (7.2%). Most encountered device related AE with Micra LP were capturing problem (37.8%), pacing problem (11.5%), and sensing problem (9.3%). Frequencies of all the analyzed AE are shown in Figure 1. The reporting of pericardial effusion (ROR 2.84, 95% CI 2.18-3.71), and dislodgment (ROR 1.85, 95% CI 1.26-2.73) were significantly higher with Aveir, whereas cardiac arrest (ROR 0.18, 95% CI 0.04-0.74) was disproportionately lower. Overall, patient related AE were significantly higher (ROR 1.53, 95% CI 1.20-1.95) and device related events were significantly lower (ROR 0.65, 95% CI 0.51-0.83) with Aveir LP compared to Micra LP (Figure 2).
Conclusion: This is the first real-world comparative analysis of two leadless pacing systems available in the United States. Our analysis showed that, when compared to Micra LP, the newer Aveir LP had lower device related events but higher patient related events, largely driven by pericardial effusion. These events could be attributed to the operator learning curve and long-term data are needed to further verify these findings.
Methods: We queried the FDA Manufacturer and User Facility Device Experience (MAUDE) database between April 2022 and December 2023 to assess the safety and AE following implantation of Aveir LP. "AVIER" and "MICRA" were the key terms used to search the MAUDE database. The event types "death" and "injury" were included in our search to capture major clinical events related to the patient. Disproportionality analysis was performed using the reporting odds ratio (ROR) to compare the adverse events of Aveir LP with Micra LP. A signal to noise ratio was considered to be significant if the confidence interval (CI) did not cross the number "one".
Results: Our search resulted in 207 event reports for Aveir LP and 1969 event reports for Micra LP. Major device related adverse events with Aveir LP were capturing problem (33.8%) followed by dislodgement (16.9%), and sensing problem (7.2%). Most encountered device related AE with Micra LP were capturing problem (37.8%), pacing problem (11.5%), and sensing problem (9.3%). Frequencies of all the analyzed AE are shown in Figure 1. The reporting of pericardial effusion (ROR 2.84, 95% CI 2.18-3.71), and dislodgment (ROR 1.85, 95% CI 1.26-2.73) were significantly higher with Aveir, whereas cardiac arrest (ROR 0.18, 95% CI 0.04-0.74) was disproportionately lower. Overall, patient related AE were significantly higher (ROR 1.53, 95% CI 1.20-1.95) and device related events were significantly lower (ROR 0.65, 95% CI 0.51-0.83) with Aveir LP compared to Micra LP (Figure 2).
Conclusion: This is the first real-world comparative analysis of two leadless pacing systems available in the United States. Our analysis showed that, when compared to Micra LP, the newer Aveir LP had lower device related events but higher patient related events, largely driven by pericardial effusion. These events could be attributed to the operator learning curve and long-term data are needed to further verify these findings.
More abstracts on this topic:
Establishing functional benchmarks and pharmacological readiness of Mantarray-Engineered Heart Tissues across multiple hiPSC-derived cardiomyocyte lines
Arefin Ayesha, Kabi Neda, Gees Katharina, Kernan Kelly, Sullivan Ellie, Luttrell Shawn, Worthen Christal, Luerman Greg, Geisse Nicholas
Trends in Adverse Events of Leadless Pacemakers in The United States, Between January 2021-December 2024Lopez Rafael, Dhruva Sanket, Tomes Madris, Neuhaus John, Redberg Rita