Final ID: Sa2019
Factors Associated with Recovery of Right Ventricular Size After Transcatheter Device Closure of Isolated Atrial Septal Defects in Children
Abstract Body (Do not enter title and authors here): Introduction: Children with significant left-to-right shunting across an isolated atrial septal defect (ASD) may have important right ventricular (RV) dilation. We sought to determine factors associated with RV recovery after percutaneous device closure.
Methods: Children who underwent transcatheter device closure of an isolated ASD were included if they had no underlying genetic defect and echocardiography from prior to, immediately after and at follow-up. General linear regression modeling was used to determine factors associated with time-related RV dimensional recovery.
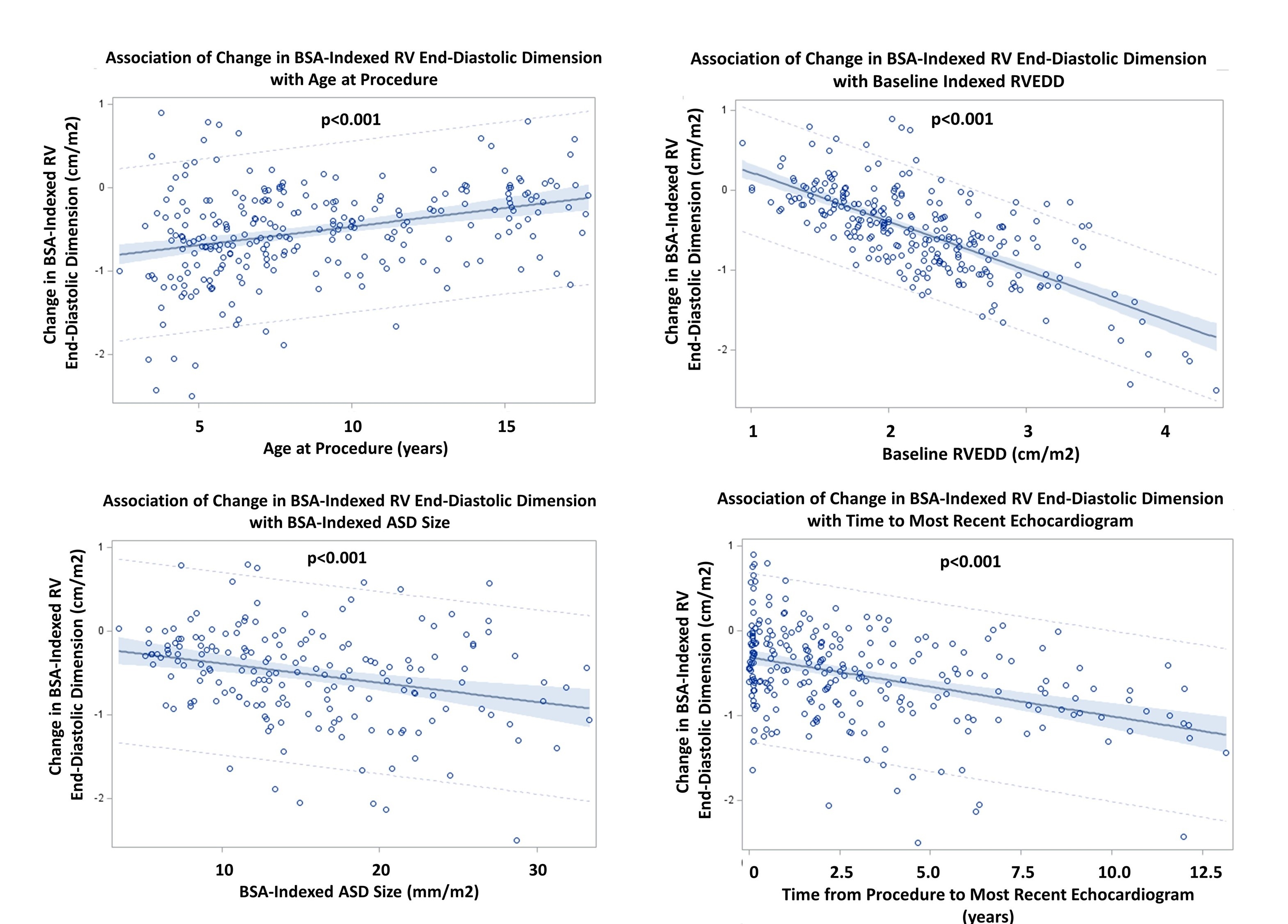
Results: Inclusion criteria were met for 385 children (62% females; mean age 8.9+4.3 years) whose procedure occurred from 11/1985 to 11/2012. A single defect was present for 85%, with a mean size indexed to BSA of 14.9+6.6 mm/m2. A single device (Amplatzer for 80%) was used for 97% of the children, with a mild residual shunt present in 14% at the procedure. Mean BSA-indexed RV end-diastolic dimension (RVEDD) immediately post-closure was 2.20+0.66 cm/m2 and decreased at a median follow-up of 1.8 years (range up to 13 years) to 1.71+0.48 cm/m2 (p<0.001), corresponding to a mean BSA-adjusted Z score of +1.51 at follow-up (>+2 for 47% of children). Significant factors associated with a greater reduction in BSA-indexed RV size (Figure) included younger age at closure (p<0.001), larger indexed ASD size (p<0.001), greater RV size at closure (p<0.001) and longer follow-up time (p<0.001). Reduction was not associated with the presence of any residual shunt post-closure. QRS duration on ECG did not change significantly from pre-closure (mean 90+12 mmsec) to latest ECG (mean 89+12; p=035).
Conclusions: Despite progressive reductions in RV size after ASD device closure, some children may continue to have RV enlargement. Earlier closure is associated with greater improvements.
Methods: Children who underwent transcatheter device closure of an isolated ASD were included if they had no underlying genetic defect and echocardiography from prior to, immediately after and at follow-up. General linear regression modeling was used to determine factors associated with time-related RV dimensional recovery.
Results: Inclusion criteria were met for 385 children (62% females; mean age 8.9+4.3 years) whose procedure occurred from 11/1985 to 11/2012. A single defect was present for 85%, with a mean size indexed to BSA of 14.9+6.6 mm/m2. A single device (Amplatzer for 80%) was used for 97% of the children, with a mild residual shunt present in 14% at the procedure. Mean BSA-indexed RV end-diastolic dimension (RVEDD) immediately post-closure was 2.20+0.66 cm/m2 and decreased at a median follow-up of 1.8 years (range up to 13 years) to 1.71+0.48 cm/m2 (p<0.001), corresponding to a mean BSA-adjusted Z score of +1.51 at follow-up (>+2 for 47% of children). Significant factors associated with a greater reduction in BSA-indexed RV size (Figure) included younger age at closure (p<0.001), larger indexed ASD size (p<0.001), greater RV size at closure (p<0.001) and longer follow-up time (p<0.001). Reduction was not associated with the presence of any residual shunt post-closure. QRS duration on ECG did not change significantly from pre-closure (mean 90+12 mmsec) to latest ECG (mean 89+12; p=035).
Conclusions: Despite progressive reductions in RV size after ASD device closure, some children may continue to have RV enlargement. Earlier closure is associated with greater improvements.
More abstracts on this topic:
A Meta-Analysis Comparing Same-Day Discharge to Later-Day Discharge in Transcatheter Aortic Valve Replacement
Jain Hritvik, Passey Siddhant, Jain Jyoti, Goyal Aman, Wasir Amanpreet, Ahmed Mushood, Patel Nandan, Yadav Ashish, Shah Janhvi, Mehta Aryan
A rare case of ventriculobronchial fistula caused by an epicardial defibrillator patchAlampoondi Venkataramanan Sai Vikram, Windle John