Final ID: Mo2168
THE TWO-HIT HYPOTHESIS?: NEW ONEST SEVERE CARDIOMYOPATHY AND CARDIAC ARRYTHMIA IN A HIGH RISK PATIENT WITH NEUROGENIC ORTHOSTATIC HYPOTENSION ON DROXIDOPA
Abstract Body (Do not enter title and authors here): Introduction
Droxidopa (DD) is the second FDA approved drug after midodrine for neurogenic orthostatic hypotension (nOH). Isaacon et al reported that 19 (5.4%) of total 350 patients on droxidopa had 25 cardiac events, most commonly atrial fibrillation in a mean duration of 363 days. Currently, limited long term cardiovascular safety data is available. We encountered a patient with nOH on DD, who developed symptomatic frequent premature ventricular contractions (PVCs) and severe cardiomyopathy (CM).
Case
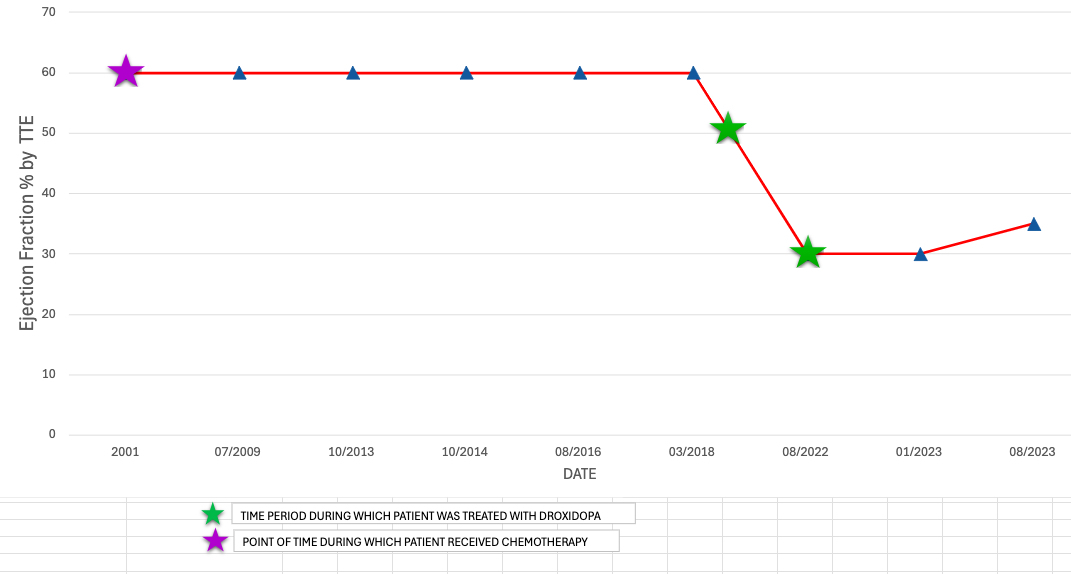
A 52-year-old woman with a long-standing nOH had persistent lightheadedness, dizziness, presyncope and hypotension despite midodrine, fludrocortisone, and non-pharmacological measures. Her past medical history was significant for nodular lymphocyte dominant Hodgkin lymphoma status post AVBD chemotherapy in 2001, with recurrence in 2012, which was treated with autologous stem cell transplantation.
Patient was switched to DD from midodrine for nOH and responded well. However, she developed frequent PVCs(13%), left bundle branch block(QRS 127 ms), with new onset CM approximately 2 years later. The ischemic work-up with coronary angiography was normal and cardiac MRI showed no late gadolinium enhancement. DD was stopped and patient was started on GDMT for HFrEF with subsequent improvement in PVC burden(1%). GDMT uptitration was limited due to hypotension. Without EF recovery up to 2 years, she received CRT-D placement.
Discussion
CM after anthracycline therapy is rare after 5 years. Our patient’s last exposure to doxorubincin was 17 years before the onset of CM. Serial echocardiograms showed normal cardiac function over years. A potential association of developing takotsubo cardiomyopathy was suggested in patients on DD by Sato et al, the potential mechanism being the increased adrenergic drive. The rapid development of non-ischemic, non-infiltrative CM and cardiac arrythmia, which subsequently improved by stopping the DD, is alarming for DD’s potential side effect.
Conclusion
Caution should be taken in prescribing DD in patients with nOH, who are at higher risk for development of cardiac arrythmia and cardiomyopathy. Further studies and data are needed in identifying or excluding the potential cardiovascular side effects.
Droxidopa (DD) is the second FDA approved drug after midodrine for neurogenic orthostatic hypotension (nOH). Isaacon et al reported that 19 (5.4%) of total 350 patients on droxidopa had 25 cardiac events, most commonly atrial fibrillation in a mean duration of 363 days. Currently, limited long term cardiovascular safety data is available. We encountered a patient with nOH on DD, who developed symptomatic frequent premature ventricular contractions (PVCs) and severe cardiomyopathy (CM).
Case
A 52-year-old woman with a long-standing nOH had persistent lightheadedness, dizziness, presyncope and hypotension despite midodrine, fludrocortisone, and non-pharmacological measures. Her past medical history was significant for nodular lymphocyte dominant Hodgkin lymphoma status post AVBD chemotherapy in 2001, with recurrence in 2012, which was treated with autologous stem cell transplantation.
Patient was switched to DD from midodrine for nOH and responded well. However, she developed frequent PVCs(13%), left bundle branch block(QRS 127 ms), with new onset CM approximately 2 years later. The ischemic work-up with coronary angiography was normal and cardiac MRI showed no late gadolinium enhancement. DD was stopped and patient was started on GDMT for HFrEF with subsequent improvement in PVC burden(1%). GDMT uptitration was limited due to hypotension. Without EF recovery up to 2 years, she received CRT-D placement.
Discussion
CM after anthracycline therapy is rare after 5 years. Our patient’s last exposure to doxorubincin was 17 years before the onset of CM. Serial echocardiograms showed normal cardiac function over years. A potential association of developing takotsubo cardiomyopathy was suggested in patients on DD by Sato et al, the potential mechanism being the increased adrenergic drive. The rapid development of non-ischemic, non-infiltrative CM and cardiac arrythmia, which subsequently improved by stopping the DD, is alarming for DD’s potential side effect.
Conclusion
Caution should be taken in prescribing DD in patients with nOH, who are at higher risk for development of cardiac arrythmia and cardiomyopathy. Further studies and data are needed in identifying or excluding the potential cardiovascular side effects.
More abstracts on this topic:
A Cardiac Ryanodine Receptor C-terminal Truncation Causes Calcium Release Deficiency Syndrome, but not Catecholaminergic Polymorphic Ventricular Tachycardia
Tian Shanshan, Ni Mingke, Wang Hui, Zhu Hai-lei, Wang Ruiwu, Estillore John Paul, Chen Wayne
A peptoid derivative of alpha-calcitonin gene related peptide improves cardiac function in pressure-overload heart failure miceKumar Ambrish, Deloach Sarah, Dipette Donald, Potts Jay