Final ID: Su1060
Optimal threshold of donor-derived cell-free DNA for detection of cardiac transplant rejection: a meta-analysis
Abstract Body (Do not enter title and authors here): Background
Plasma donor-derived cell-free DNA (dd-cfDNA) has emerged as a biomarker for acute heart transplant rejection as a potential adjunct to endomyocardial biopsy (EMBx). However, an established threshold for dd-cfDNA in acute cardiac transplant rejection is lacking, with prior studies employing different study specific thresholds. This meta-analysis aims to assess the overall diagnostic accuracy of dd-cfDNA across various cutoffs and provide an optimal threshold.
Method
PubMed and Web of Science were searched for relevant publications reporting on the use of dd-cfDNA for the detection of acute cardiac transplant rejection defined as acute cellular rejection (ACR) and/or antibody-mediated rejection (AMR), with EMBx as the reference standard. Pooled sensitivity and specificity were estimated across different thresholds of dd-cfDNA across studies. The optimal dd-cfDNA threshold was estimated by specificity and 1-sensitivity in each data point pooled with fractional polynomial models, maximizing the Youden index between these models.
Results
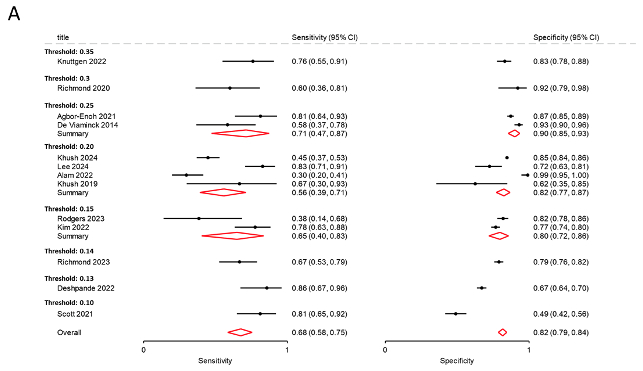
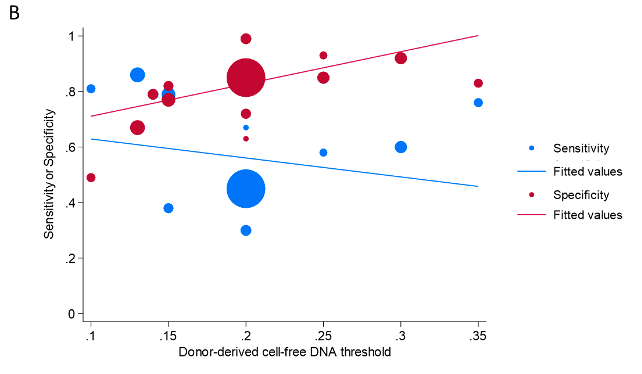
Out of 247 screened papers, 13 studies were included, comprising 12,017 samples from 3,723 patients. Twelve studies used both ACR and AMR and one study used only ACR for the definition of transplant rejection. The thresholds of dd-cfDNA varied across studies, ranging from 0.1 to 0.35 (Figure A). The overall pooled sensitivity and specificity for detecting acute transplant rejection were 0.68 (95% CI: 0.58-0.77) and 0.82 (95% CI: 0.74-0.88) respectively. The areas under the hierarchical modeling-based summary receiver-operating characteristics (sROC) curves were 0.82 (95% CI: 0.78-0.85). Specificity increased with higher thresholds while sensitivity decreased (Figure A and B). The optimal dd-cfDNA threshold derived from combining all included studies was 0.218.
Conclusion
Dd-cfDNA assay demonstrates high diagnostic accuracy for acute cardiac transplant rejection, particularly notable for its high specificity. However, there exists significant inconsistency in dd-cfDNA thresholds across studies. Increasing the threshold leads to a trade-off between increased specificity and decreased sensitivity. A cutoff of 0.22 may be considered for dd-cfDNA screening for heart transplant rejection.
Plasma donor-derived cell-free DNA (dd-cfDNA) has emerged as a biomarker for acute heart transplant rejection as a potential adjunct to endomyocardial biopsy (EMBx). However, an established threshold for dd-cfDNA in acute cardiac transplant rejection is lacking, with prior studies employing different study specific thresholds. This meta-analysis aims to assess the overall diagnostic accuracy of dd-cfDNA across various cutoffs and provide an optimal threshold.
Method
PubMed and Web of Science were searched for relevant publications reporting on the use of dd-cfDNA for the detection of acute cardiac transplant rejection defined as acute cellular rejection (ACR) and/or antibody-mediated rejection (AMR), with EMBx as the reference standard. Pooled sensitivity and specificity were estimated across different thresholds of dd-cfDNA across studies. The optimal dd-cfDNA threshold was estimated by specificity and 1-sensitivity in each data point pooled with fractional polynomial models, maximizing the Youden index between these models.
Results
Out of 247 screened papers, 13 studies were included, comprising 12,017 samples from 3,723 patients. Twelve studies used both ACR and AMR and one study used only ACR for the definition of transplant rejection. The thresholds of dd-cfDNA varied across studies, ranging from 0.1 to 0.35 (Figure A). The overall pooled sensitivity and specificity for detecting acute transplant rejection were 0.68 (95% CI: 0.58-0.77) and 0.82 (95% CI: 0.74-0.88) respectively. The areas under the hierarchical modeling-based summary receiver-operating characteristics (sROC) curves were 0.82 (95% CI: 0.78-0.85). Specificity increased with higher thresholds while sensitivity decreased (Figure A and B). The optimal dd-cfDNA threshold derived from combining all included studies was 0.218.
Conclusion
Dd-cfDNA assay demonstrates high diagnostic accuracy for acute cardiac transplant rejection, particularly notable for its high specificity. However, there exists significant inconsistency in dd-cfDNA thresholds across studies. Increasing the threshold leads to a trade-off between increased specificity and decreased sensitivity. A cutoff of 0.22 may be considered for dd-cfDNA screening for heart transplant rejection.
More abstracts on this topic:
A Simple One-Item Nursing Falls Assessment Predicts Outcomes For Patients With Stage D Heart Failure Undergoing Surgical Advanced Therapies
Salvador Vincent, Perez Jaime Abraham, Hudec Paige, Gorodeski Eiran, Oneill Thomas
A validated metabolite-based biomarker score for fruit and vegetable intake and associations with all-cause mortality and incident cardiometabolic diseasesOude Griep Linda, Li Chunxiao, Koulman Albert, Imamura Fumiaki, Wareham Nicholas, Forouhi Nita