Final ID: 4144964
Low-Dose IL-2 Lowers Arterial Inflammation and Trends Towards Lower MACE in patients with ACS: The results of the IVORY trial and IVORY FINALE study
Abstract Body (Do not enter title and authors here): Major adverse cardiovascular (CV) events (MACE) occur in a substantioal percentage of individuals following acute coronary syndromes (ACS), primarily driven by residual vascular inflammation. Anti-inflammatory drugs have improved CV outcomes but their use is limited by significant side-effects. Low-dose interleukin 2 (IL2) increases regulatory T (Treg) cells, which are powerful endogenous regulators of the immune response, and could provide a new, targeted anti-inflammatory strategy in high-risk ACS patients. In IVORY, we hypothesised that treatment with low-dose IL2 would reduce arterial inflammation measured by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), compared to placebo. In IVORY FINALE we hypothesised that low dose IL2 could reduce MACE compared to placebo at follow up (up to 5 years.)
IVORY was a double-blind, placebo-controlled, Phase IIb trial randomising ACS patients with high-sensitivity CRP levels ≧ 2mg/L to receive either 1.5×106IU IL2 or placebo (1:1). Dosing consisted of a daily induction (5 days) and a weekly maintenance phase (7 weeks). 18F-FDG-PET/CT imaging of the ascending aorta and carotid arteries was performed before and after treatment. The primary outcome was the difference in the mean maximum target-to-background ratio (TBRmax) in the index vessel on follow-up imaging between the groups.
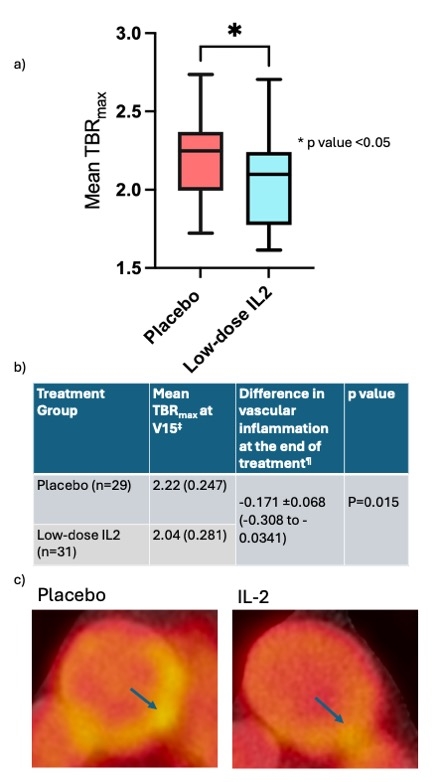
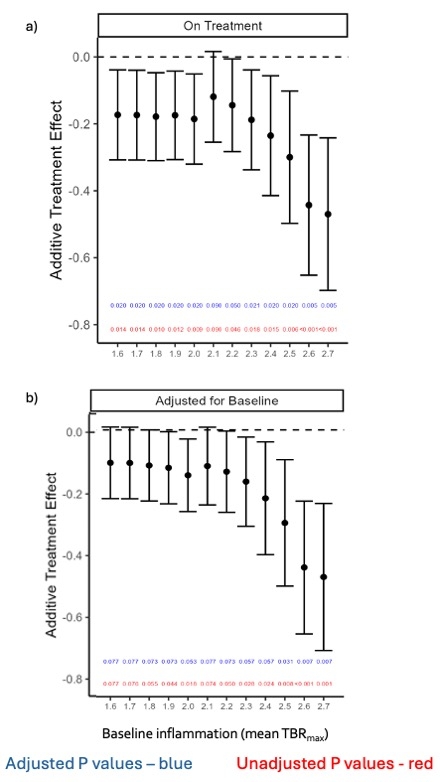
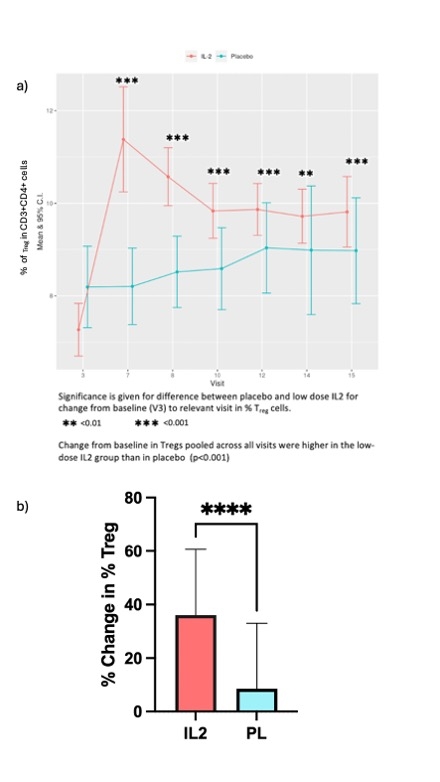
60 patients (IL2:placebo, n=31:29) completed the trial. Arterial inflammation in the index vessel was lower at the end of treatment in the IL2 group than in placebo (TBRmax = -0.171[-7.7%], 95% CI -0.308 to -0.034, p=0.015)[Fig1]. In more inflamed areas with a mean TBRmax ≧ 2 (active slices), the difference between the groups was greater (-0.185 [-8.3%], P=0.009, 95% CI -0.323 to -0.0478). Overall, the additive treatment effect of low-dose IL2 was greater at higher baseline inflammation [Fig 2]. Low-dose IL2 significantly increased circulating Tregs compared to placebo (p<0.001) [Fig 3]. There was no difference between the groups for changes in effector T cells. It was well-tolerated in ACS. At a median follow-up of 2.5 years, a trend towards lower MACE in the IL2 group (number of events [n]=0), compared to placebo (n= 7; 2 deaths, 2 non-fatal MIs, 3 unplanned revascularisations) was seen.
In summary, in high-risk ACS, immunomodulation with low-dose IL2 produces a safe and significant reduction in arterial inflammation, compared to placebo. Larger trials are needed to confirm its impact on CV outcomes.
IVORY was a double-blind, placebo-controlled, Phase IIb trial randomising ACS patients with high-sensitivity CRP levels ≧ 2mg/L to receive either 1.5×106IU IL2 or placebo (1:1). Dosing consisted of a daily induction (5 days) and a weekly maintenance phase (7 weeks). 18F-FDG-PET/CT imaging of the ascending aorta and carotid arteries was performed before and after treatment. The primary outcome was the difference in the mean maximum target-to-background ratio (TBRmax) in the index vessel on follow-up imaging between the groups.
60 patients (IL2:placebo, n=31:29) completed the trial. Arterial inflammation in the index vessel was lower at the end of treatment in the IL2 group than in placebo (TBRmax = -0.171[-7.7%], 95% CI -0.308 to -0.034, p=0.015)[Fig1]. In more inflamed areas with a mean TBRmax ≧ 2 (active slices), the difference between the groups was greater (-0.185 [-8.3%], P=0.009, 95% CI -0.323 to -0.0478). Overall, the additive treatment effect of low-dose IL2 was greater at higher baseline inflammation [Fig 2]. Low-dose IL2 significantly increased circulating Tregs compared to placebo (p<0.001) [Fig 3]. There was no difference between the groups for changes in effector T cells. It was well-tolerated in ACS. At a median follow-up of 2.5 years, a trend towards lower MACE in the IL2 group (number of events [n]=0), compared to placebo (n= 7; 2 deaths, 2 non-fatal MIs, 3 unplanned revascularisations) was seen.
In summary, in high-risk ACS, immunomodulation with low-dose IL2 produces a safe and significant reduction in arterial inflammation, compared to placebo. Larger trials are needed to confirm its impact on CV outcomes.
More abstracts on this topic:
Angiotensin-converting enzyme (ACE): A new role to regulate β-oxidation in monocytic cells
Cao Duo-yao
A First-In-Human Phase 1 Study of the Safety, Tolerability, and Pharmacodynamics of REGN7544, a Novel Natriuretic Peptide Receptor 1–Blocking Monoclonal AntibodyAhmed Mohsin, Morton Lori, Olenchock Benjamin, Herman Gary, Wynne Chris, Marin Ethan, Tuckwell Katie, Xu Meng, Cheng Xiping, Redington Emily, Koyani Bharatkumar, Mateo Katrina, Thakur Mazhar, Devalaraja-narashimha Kishor