Final ID: MDP181
Phase 2 Open-label, Single-arm, Multi-center Clinical Trial to Evaluate the Efficacy and Safety of Camostat Mesylate in Patients with Protein-losing Enteropathy after Fontan Operation-Preliminary Outcome
Abstract Body (Do not enter title and authors here): Introduction
Protein-losing enteropathy (PLE) is a multifaceted condition that profoundly affects the systemic health and quality of life of Fontan patients. Despite medical progress, the treatment of PLE remains a significant challenge. This study investigates the efficacy and safety of Camostat Mesylate for managing PLE patients who have undergone the Fontan operation.
Hypothesis
We hypothesize that Camostat Mesylate will enhance the gut environment, resulting in increase of serum albumin levels and decrease of stool alpha-1 antitrypsin levels in PLE patients following Fontan operation.
Methods
This phase 2, multicenter, open-label, single-arm trial included patients over 4 years old diagnosed with PLE following Fontan operation. Camostat Mesylate was added to conventional treatments, with follow-up assessments at 1, 3, and 6 months, and a final evaluation one month after discontinuation. Efficacy was measured by changes in serum albumin, stool alpha-1 antitrypsin levels, and PLE symptoms such as diarrhea, edema, weight changes, and ascites.
Results
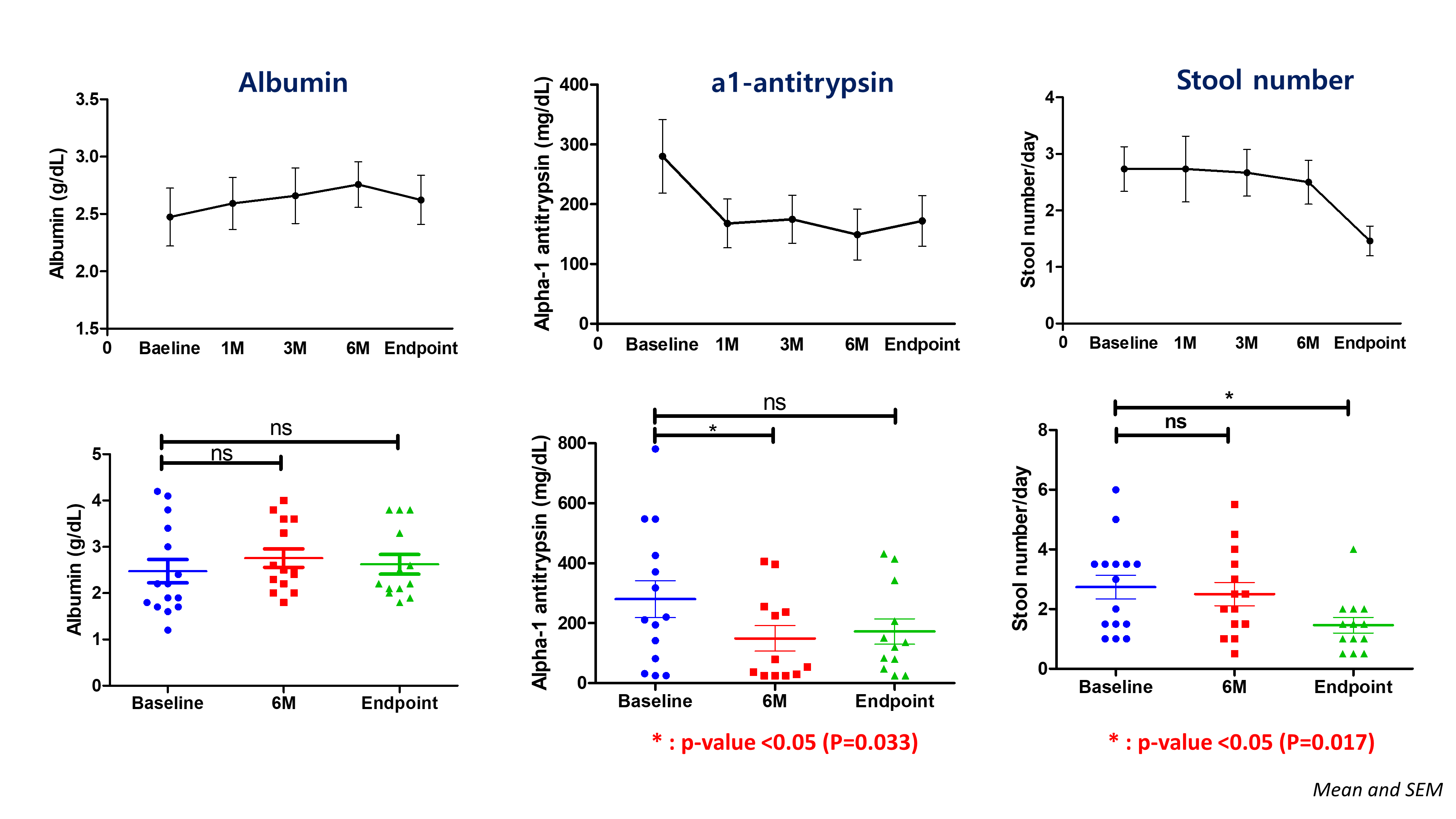
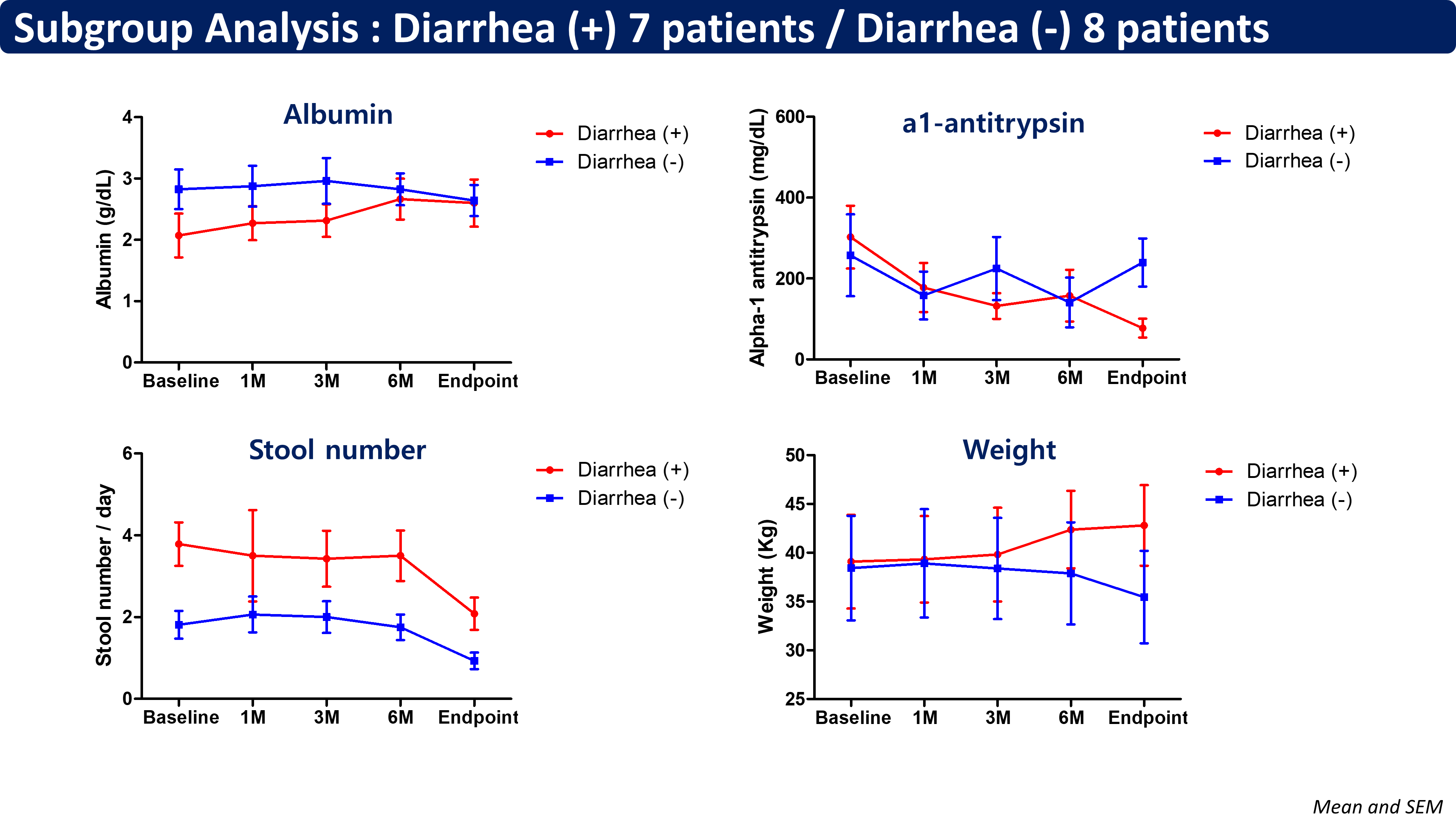
Nineteen patients were enrolled in the study, of whom fifteen patients completed follow-up as per protocol. The median age was 15 years (interquartile range, 12.0-21.3). The median time between the Fontan operation and PLE diagnosis was 2.4 years. Serum albumin levels increased from 2.5 to 2.6 g/dL (p=0.504), and stool alpha-1 antitrypsin levels decreased significantly from 280.0 to 172.1 mg/dL (p=0.033). Notably, patients with diarrhea at baseline showed substantial improvement in both parameters, with increased serum albumin levels from 1.8 to 2.2 g/dL and decreased stool alpha-1 antitrypsin levels from 220.3 to 80.2 mg/dL. No serious adverse events were reported during study period.
Conclusions
Camostat Mesylate demonstrated safety and efficacy, reducing stool alpha-1 antitrypsin in PLE patients after Fontan operation, especially those with diarrhea at baseline. Therefore, Camostat Mesylate could be considered as an additional treatment option for patients with PLE following Fontan operation.
Key words
Camostat mesylate; protein-losing enteropathy; Fontan operation
Source of Funding
This research was funded by SNUH Lee Kun-hee Child Cancer & Rare Disease Project, Republic of Korea.
Protein-losing enteropathy (PLE) is a multifaceted condition that profoundly affects the systemic health and quality of life of Fontan patients. Despite medical progress, the treatment of PLE remains a significant challenge. This study investigates the efficacy and safety of Camostat Mesylate for managing PLE patients who have undergone the Fontan operation.
Hypothesis
We hypothesize that Camostat Mesylate will enhance the gut environment, resulting in increase of serum albumin levels and decrease of stool alpha-1 antitrypsin levels in PLE patients following Fontan operation.
Methods
This phase 2, multicenter, open-label, single-arm trial included patients over 4 years old diagnosed with PLE following Fontan operation. Camostat Mesylate was added to conventional treatments, with follow-up assessments at 1, 3, and 6 months, and a final evaluation one month after discontinuation. Efficacy was measured by changes in serum albumin, stool alpha-1 antitrypsin levels, and PLE symptoms such as diarrhea, edema, weight changes, and ascites.
Results
Nineteen patients were enrolled in the study, of whom fifteen patients completed follow-up as per protocol. The median age was 15 years (interquartile range, 12.0-21.3). The median time between the Fontan operation and PLE diagnosis was 2.4 years. Serum albumin levels increased from 2.5 to 2.6 g/dL (p=0.504), and stool alpha-1 antitrypsin levels decreased significantly from 280.0 to 172.1 mg/dL (p=0.033). Notably, patients with diarrhea at baseline showed substantial improvement in both parameters, with increased serum albumin levels from 1.8 to 2.2 g/dL and decreased stool alpha-1 antitrypsin levels from 220.3 to 80.2 mg/dL. No serious adverse events were reported during study period.
Conclusions
Camostat Mesylate demonstrated safety and efficacy, reducing stool alpha-1 antitrypsin in PLE patients after Fontan operation, especially those with diarrhea at baseline. Therefore, Camostat Mesylate could be considered as an additional treatment option for patients with PLE following Fontan operation.
Key words
Camostat mesylate; protein-losing enteropathy; Fontan operation
Source of Funding
This research was funded by SNUH Lee Kun-hee Child Cancer & Rare Disease Project, Republic of Korea.
More abstracts on this topic:
Aerobic Capacity of Adults with Fontan Palliation: Disease-specific Reference Values and Relationship to Outcomes
Ali Ahmed, Goda Ahmed, Abozied Omar, Egbe Alexander
Advanced Complementary Imaging Analysis of Single Ventricle Patients Using Cardiac Magnetic Resonance and EchocardiographyNaum Athanasios, Loke Yue-hin, Meyers Brett, Payne Ronald, Vlachos Pavlos