Final ID: Sa2188
Benefits and Risks Associated with Mineralocorticoid Receptor Antagonists in Tafamidis-treated Transthyretin Amyloid Cardiomyopathy: A Real-World Analysis
Abstract Body (Do not enter title and authors here): Background
There is currently no data on the use of mineralocorticoid receptor antagonists (MRA) in tafamidis-treated transthyretin amyloid cardiomyopathy (ATTR-CM).
Research Question
Does the addition of MRA provide extra benefits in ATTR-CM patients treated with tafamidis?
Aim
To assess the benefits and risks associated with MRA in tafamidis-treated ATTR-CM through a new-user design propensity-score matching (PSM) analysis.
Methods
We conducted a retrospective, PSM cohort study using the TriNetX Global database. We included adult ATTR-CM patients who had their first tafamidis prescription within 1 year after the identification of amyloidosis between January 1, 2018, and May 1, 2023. MRA users and non-users were subsequently identified, with the former defined as those having both tafamidis and spironolactone/eplerenone, and the latter having tafamidis without MRA. Patients with prior MRA use before their first tafamidis prescription were excluded. The primary endpoint was major adverse cardiac events (MACE), defined as a composite of all-cause mortality, heart failure (HF) hospitalization, or cardiac arrest/ventricular tachycardia/fibrillation. Secondary endpoints were individual MACE. The safety endpoint was a composite of acute kidney injury (AKI) or hyperkalemia. Incident gastrointestinal bleeding and pneumonia were set as falsification endpoints. We compare study endpoints occurring within two years of tafamidis initiation between MRA users and non-users.
Results
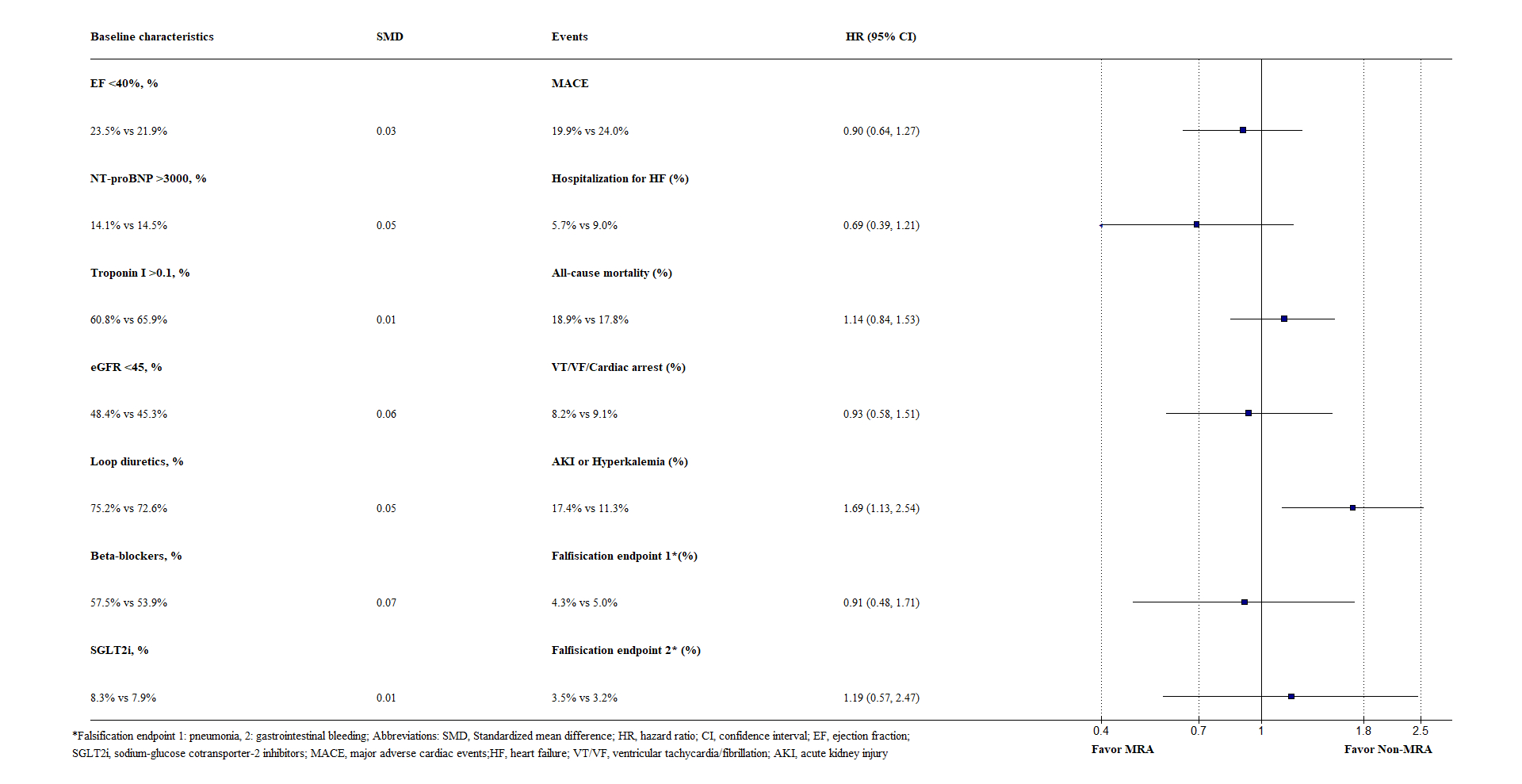
Among 2,174 tafamidis-treated ATTR-CM patients, 471 MRA users were matched to 471 non-users with similar age (76.7 vs. 76.7), sex (female, 16.8% vs. 17.4%), and mean ejection fraction (50.1% vs. 49.9%). Amyloid staging markers and important medications were also balanced (Figure). MRA use was not associated with a significant reduction in MACE (HR, 0.90; 95% CI, 0.64 to 1.27) (Figure). Conversely, it was associated with a significant increase in AKI or hyperkalemia (HR, 1.69; 95% CI, 1.13 to 2.54) (Figure). The two falsification endpoints were comparable between the two groups (Figure).
Conclusions
In ATTR-CM patients treated with tafamidis, MRA was not associated with a significant reduction in MACE but with an increased risk of AKI or hyperkalemia.
There is currently no data on the use of mineralocorticoid receptor antagonists (MRA) in tafamidis-treated transthyretin amyloid cardiomyopathy (ATTR-CM).
Research Question
Does the addition of MRA provide extra benefits in ATTR-CM patients treated with tafamidis?
Aim
To assess the benefits and risks associated with MRA in tafamidis-treated ATTR-CM through a new-user design propensity-score matching (PSM) analysis.
Methods
We conducted a retrospective, PSM cohort study using the TriNetX Global database. We included adult ATTR-CM patients who had their first tafamidis prescription within 1 year after the identification of amyloidosis between January 1, 2018, and May 1, 2023. MRA users and non-users were subsequently identified, with the former defined as those having both tafamidis and spironolactone/eplerenone, and the latter having tafamidis without MRA. Patients with prior MRA use before their first tafamidis prescription were excluded. The primary endpoint was major adverse cardiac events (MACE), defined as a composite of all-cause mortality, heart failure (HF) hospitalization, or cardiac arrest/ventricular tachycardia/fibrillation. Secondary endpoints were individual MACE. The safety endpoint was a composite of acute kidney injury (AKI) or hyperkalemia. Incident gastrointestinal bleeding and pneumonia were set as falsification endpoints. We compare study endpoints occurring within two years of tafamidis initiation between MRA users and non-users.
Results
Among 2,174 tafamidis-treated ATTR-CM patients, 471 MRA users were matched to 471 non-users with similar age (76.7 vs. 76.7), sex (female, 16.8% vs. 17.4%), and mean ejection fraction (50.1% vs. 49.9%). Amyloid staging markers and important medications were also balanced (Figure). MRA use was not associated with a significant reduction in MACE (HR, 0.90; 95% CI, 0.64 to 1.27) (Figure). Conversely, it was associated with a significant increase in AKI or hyperkalemia (HR, 1.69; 95% CI, 1.13 to 2.54) (Figure). The two falsification endpoints were comparable between the two groups (Figure).
Conclusions
In ATTR-CM patients treated with tafamidis, MRA was not associated with a significant reduction in MACE but with an increased risk of AKI or hyperkalemia.
More abstracts on this topic:
Acoramidis Reduces All-Cause Mortality (ACM) and Cardiovascular-Related Hospitalization (CVH): Initial Outcomes From the ATTRibute-CM Open-Label Extension (OLE) Study
Judge Daniel, Masri Ahmad, Obici Laura, Poulsen Steen, Sarswat Nitasha, Shah Keyur, Soman Prem, Cao Xiaofan, Wang Kevin, Pecoraro Maria, Tamby Jean-francois, Gillmore Julian, Katz Leonid, Fox Jonathan, Maurer Mathew, Alexander Kevin, Ambardekar Amrut, Cappelli Francesco, Fontana Marianna, Garcia-pavia Pablo, Grogan Martha, Hanna Mazen
A diagnostic challenge overcome with persistent clinical suspicion in a case of cardiac AL amyloidosisZimmerman Allison, Kuriakose Philip, Godfrey Amanda, Ananthasubramaniam Karthikeyan, Cowger Jennifer, Al-darzi Waleed