Final ID: MDP91
O2-independent photodynamic neuroimmune modulation for prevention and treatment of malignant arrhythmia post myocardial infarction
Abstract Body (Do not enter title and authors here): Background
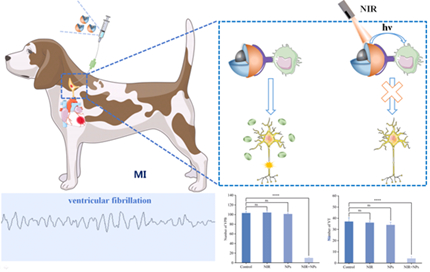
Hyperactivation of the left stellate ganglion (LSG) is a key link in the occurrence of ventricular arrhythmias after myocardial infarction (MI). It is reported that neuroimmune interaction based on the depleting of macrophages modulated the overactive neural activity. However, exogenous macrophage scavengers, which is the common depletion strategy in animal models, are hardly capable of depleting the target cells selectively in certain tissues and transient control performance. Consequently, a degradable nanocomposite (PPSM@CS/DSS) were fabricatedto deplete M1 macrophages selectively in LSG and further inhibit the overactive LSG neural activity after myocardial infarction.
Hypothesis
In this study, we constructed a degradable nanocomposite with dual functions of targeting M1 macrophages and oxygen-independent PDT-mediated neuroimmune modulation, which isanticipated to deplete M1 macrophages selectively in LSG and further inhibit the overactive LSG neural activity after myocardial infarctionfor prevention and treatment of ventricular arrhythmias post MI.
Methods
The prepared nanocomposite material, which is capable of targeting M1 macrophages and oxygen-independent PDT-mediated neuroimmune modulation, was slowly microinjected into LSG of Beagle dogs. The effectiveness and safety of this method based on apoptosisof M1 macrophagesby oxidizing active species was explored and the mechanism of prevention as well as treatment of malignant arrhythmias were discussed. M1 macrophages were selectively apoptotic in the LSG after myocardial infarction under the irradiation of near infrared light.
Results
PPSM@CS/DSS is a core-shell structure with a particle size of about 50nm. The PPSM@CS/DSS nanocomposites exhibits band adsorption between 200-900 nm with a pronounced peaks at 650 nm.Cell experiments showed that PPSM@CS/DS was targeted and mainly induced apoptosis of M1 macrophages under 650nm near-red light, but did not significantly increase apoptosis of neuronal cells. PPSM@CS/DSS significantly reduced LSG activity and the incidence of malignant arrhythmias after MI in Beagle dogs under the action of 650nm light.
Conclusion
An innovative nanomaterial for regulating LSG through depletion M1 macrophages selectively in LSG is developed to prevent and treat malignant arrhythmias after myocardial infarction.The implementation of this work will provide a novel neural modulation strategy for preventing ventricular arrhythmias.
Hyperactivation of the left stellate ganglion (LSG) is a key link in the occurrence of ventricular arrhythmias after myocardial infarction (MI). It is reported that neuroimmune interaction based on the depleting of macrophages modulated the overactive neural activity. However, exogenous macrophage scavengers, which is the common depletion strategy in animal models, are hardly capable of depleting the target cells selectively in certain tissues and transient control performance. Consequently, a degradable nanocomposite (PPSM@CS/DSS) were fabricatedto deplete M1 macrophages selectively in LSG and further inhibit the overactive LSG neural activity after myocardial infarction.
Hypothesis
In this study, we constructed a degradable nanocomposite with dual functions of targeting M1 macrophages and oxygen-independent PDT-mediated neuroimmune modulation, which isanticipated to deplete M1 macrophages selectively in LSG and further inhibit the overactive LSG neural activity after myocardial infarctionfor prevention and treatment of ventricular arrhythmias post MI.
Methods
The prepared nanocomposite material, which is capable of targeting M1 macrophages and oxygen-independent PDT-mediated neuroimmune modulation, was slowly microinjected into LSG of Beagle dogs. The effectiveness and safety of this method based on apoptosisof M1 macrophagesby oxidizing active species was explored and the mechanism of prevention as well as treatment of malignant arrhythmias were discussed. M1 macrophages were selectively apoptotic in the LSG after myocardial infarction under the irradiation of near infrared light.
Results
PPSM@CS/DSS is a core-shell structure with a particle size of about 50nm. The PPSM@CS/DSS nanocomposites exhibits band adsorption between 200-900 nm with a pronounced peaks at 650 nm.Cell experiments showed that PPSM@CS/DS was targeted and mainly induced apoptosis of M1 macrophages under 650nm near-red light, but did not significantly increase apoptosis of neuronal cells. PPSM@CS/DSS significantly reduced LSG activity and the incidence of malignant arrhythmias after MI in Beagle dogs under the action of 650nm light.
Conclusion
An innovative nanomaterial for regulating LSG through depletion M1 macrophages selectively in LSG is developed to prevent and treat malignant arrhythmias after myocardial infarction.The implementation of this work will provide a novel neural modulation strategy for preventing ventricular arrhythmias.
More abstracts on this topic:
A Novel Cardioprotective Mechanism in Myocardial Reperfusion Injury: Dual Neutrophil Modulation and ROS/HOCl Scavenging by an Atypical Chemokine
Zwissler Leon, Bernhagen Juergen, Cabrera-fuentes Hector Alejandro, Hernandez Resendiz Sauri, Yap En Ping, Schindler Lisa, Zhang Zhishen, Dickerhof Nina, Hampton Mark, Liehn Elisa, Hausenloy Derek
A New Analytical Approach for Noninvasive Reconstruction of the Entire Left Ventricular Pressure Waveform in Myocardial Ischemia and InfarctionBilgi Coskun, Li Jiajun, Alavi Rashid, Dai Wangde, Matthews Ray, Kloner Robert, Pahlevan Niema