Final ID: Sa3009
Pharmacosurveillance study of FDA Adverse Event Reporting System (FAERS) events of Tirzepatide and Semaglutide
Abstract Body (Do not enter title and authors here): Background: GLP-1 Receptor Agonists have become widely used for treating Diabetes Mellitus Type 2 and weight loss in patients across the United States. However, new data reports increasing cases of pancreatitis, acute kidney injury, increased Hba1c, and thyroid malignancy.
Research question: Like many medications, GLP-1 receptor antagonists have a risk of side effects. The question is which medication, Semaglutide or Tirzepatide, has the highest risk of developing the above mentioned side effects.
Goals: Using the FAERS database to calculate the reporting odds ratio (ROR) for the risk of pancreatitis, acute kidney injury, increased HgA1c, and thyroid malignancy.
Methods: The FDA's FAERS system gathers information on adverse events and medication errors. The data shows that both Semaglutide and Tirzepatide have been linked to pancreatitis, acute kidney injury, elevation in HbA1c, and various types of thyroid malignancies. For consistent analysis, this data covers the period from 2022 to 2024 for both drugs.
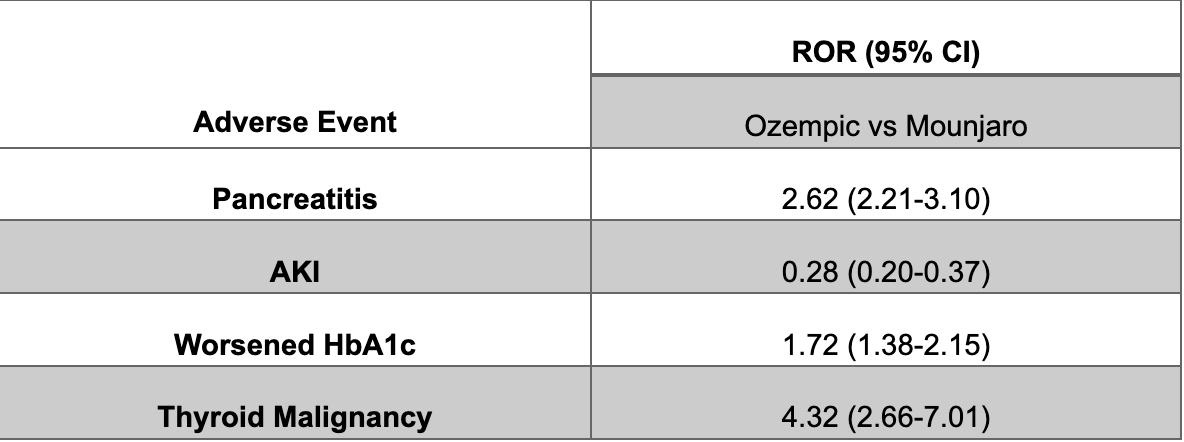
Results: January 2022- March 2024 data showed that Semaglutide and Tirzepatide had 11584 AE and 29453 AE, respectively. Semaglutide had a higher risk of developing pancreatitis, elevation of HbA1c, and thyroid malignancy compared to Tirzepatide (ROR 2.62, 95% CI 2.21-3.10) (ROR 1.72, 95% CI 1.38 to 2.15), (ROR 4.32, 95%CI 2.66 to 7.01) respectively. However, Semaglutide had 72% fewer reported cases of AKI than Semaglutide (ROR 0.28, 95% CI= 0.20 to 0.37).
Conclusion: Lately, the popularity of GLP-1 Agonists is soaring mainly due to their role in facilitating weight loss and providing additional cardiovascular benefits. To the best of our knowledge, this is the first study comparing the likelihood of these four AEs with Semaglutideand Tirzepatide, using the FAERS Database. Being the newer agent, Tirzepatide is significantly less strongly associated with three AEs than AKI. Additional studies are needed to investigate further the epidemiology and pathophysiology of AKI with Tirzepatide use. In the meantime, Tirzepatide should be the drug of choice in patients who are at high risk for pancreatitis, thyroid cancer, and poorly controlled DM. Semaglutide might be considered in chronic kidney disease patients.
Research question: Like many medications, GLP-1 receptor antagonists have a risk of side effects. The question is which medication, Semaglutide or Tirzepatide, has the highest risk of developing the above mentioned side effects.
Goals: Using the FAERS database to calculate the reporting odds ratio (ROR) for the risk of pancreatitis, acute kidney injury, increased HgA1c, and thyroid malignancy.
Methods: The FDA's FAERS system gathers information on adverse events and medication errors. The data shows that both Semaglutide and Tirzepatide have been linked to pancreatitis, acute kidney injury, elevation in HbA1c, and various types of thyroid malignancies. For consistent analysis, this data covers the period from 2022 to 2024 for both drugs.
Results: January 2022- March 2024 data showed that Semaglutide and Tirzepatide had 11584 AE and 29453 AE, respectively. Semaglutide had a higher risk of developing pancreatitis, elevation of HbA1c, and thyroid malignancy compared to Tirzepatide (ROR 2.62, 95% CI 2.21-3.10) (ROR 1.72, 95% CI 1.38 to 2.15), (ROR 4.32, 95%CI 2.66 to 7.01) respectively. However, Semaglutide had 72% fewer reported cases of AKI than Semaglutide (ROR 0.28, 95% CI= 0.20 to 0.37).
Conclusion: Lately, the popularity of GLP-1 Agonists is soaring mainly due to their role in facilitating weight loss and providing additional cardiovascular benefits. To the best of our knowledge, this is the first study comparing the likelihood of these four AEs with Semaglutideand Tirzepatide, using the FAERS Database. Being the newer agent, Tirzepatide is significantly less strongly associated with three AEs than AKI. Additional studies are needed to investigate further the epidemiology and pathophysiology of AKI with Tirzepatide use. In the meantime, Tirzepatide should be the drug of choice in patients who are at high risk for pancreatitis, thyroid cancer, and poorly controlled DM. Semaglutide might be considered in chronic kidney disease patients.
More abstracts on this topic:
A Focus for Improvement - Factors for Lab Adherence in a Pediatric Preventive Cardiology Program
Holsinger Hunter, Porterfield Ronna, Taylor Makenna, Dresbach Bethany, Seipel Brittany, Igwe Chukwuemeka, Alvarado Chance, Tran Andrew
A Focus for Improvement - Factors for Lab Adherence in a Pediatric Preventive Cardiology ProgramHolsinger Hunter, Porterfield Ronna, Taylor Makenna, Dresbach Bethany, Seipel Brittany, Igwe Chukwuemeka, Alvarado Chance, Tran Andrew