Scientific Sessions 2024
/
Critical Care Cardiology Medley
/
Pre-Hospital Antiplatelet Therapy In Patients With Out-Of-Hospital Cardiac Arrest Suspected Of Acute Coronary Syndrome
Final ID: Su4165
Pre-Hospital Antiplatelet Therapy In Patients With Out-Of-Hospital Cardiac Arrest Suspected Of Acute Coronary Syndrome
Abstract Body (Do not enter title and authors here): Background: There are currently no specific guidelines regarding pre-hospital antiplatelet therapy in patients with out-of-hospital cardiac arrest (OHCA) suspected of acute coronary syndrome (ACS).
Purpose: To evaluate efficacy and safety of pre-hospital administration of antiplatelet therapy in patients with OHCA directed to a cardiac catheterization laboratory (cath lab).
Method: Using the cath lab database of a tertiary cardiovascular center, we included consecutive patients referred for coronary angiography within 24 hours of OHCA for suspected ACS from January 2012 to January 2024. Pre-hospital antiplatelet treatment was defined as prescribing any antiplatelet therapy before cath lab admission (aspirin alone or combined with a P2Y12 inhibitor). The outcomes of interest were: 1) all-cause death at 30 days, 2) intra-hospital major adverse cardiovascular events (MACE), defined by the composite of all-cause death, myocardial infarction, or stroke, and 3) intra-hospital major bleedings (BARC ≥ 3). To help account for the non-randomized pre-hospital antiplatelet administration, an Inverse Probability Weighting (IPW) approach was used to compare outcomes between the two groups.
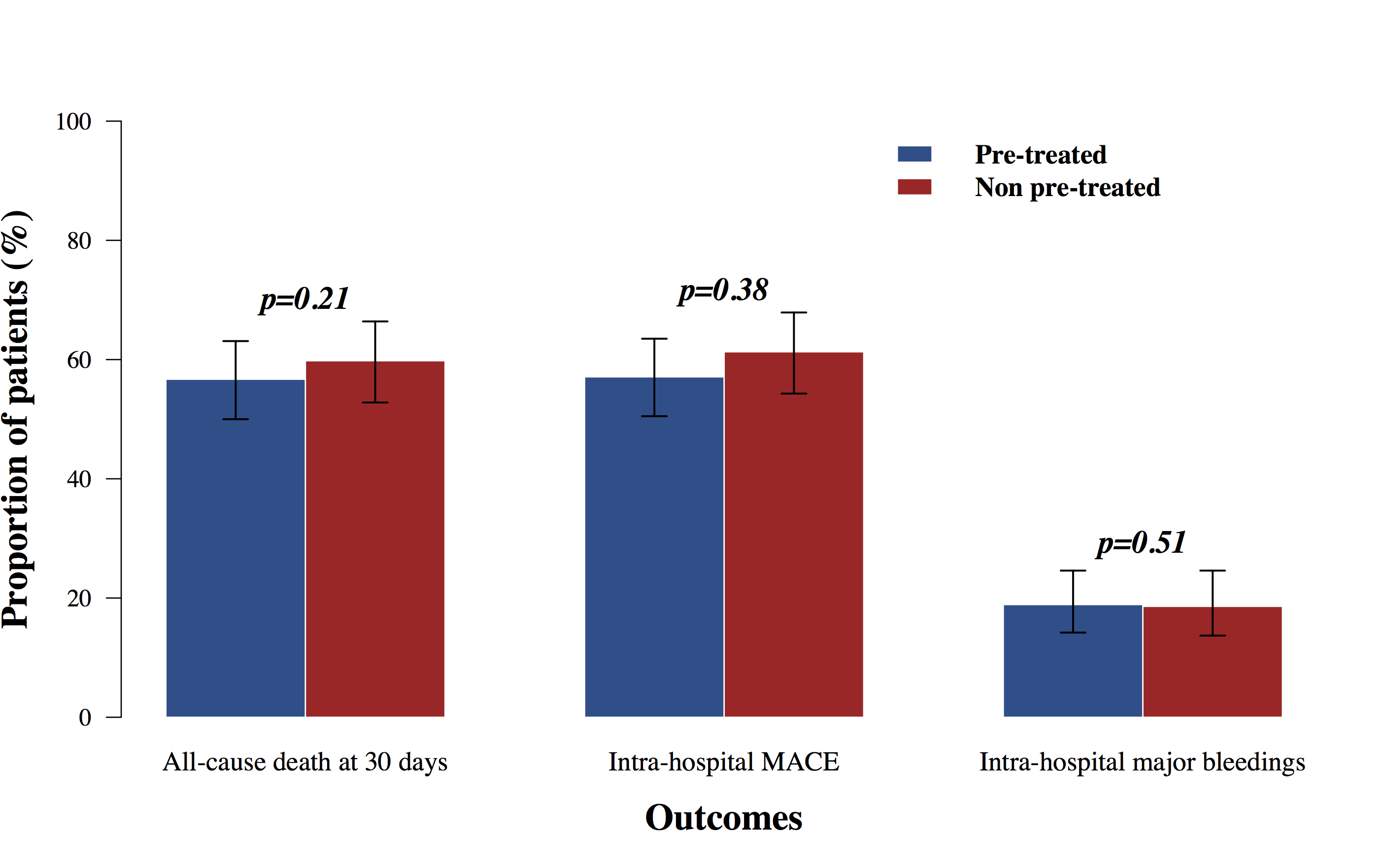
Result: Between January 2012 and January 2024, n = 411 patients with OHCA were referred to the cath lab, of whom 217 (52.8%) received a pre-treatment. Pre-hospital antiplatelet administration did not show a reduced risk of death from any cause at 30 days (56.7% [50.0%;63.1%] vs 59.8% [52.8%;66.4%], p=0.21) (Figure 1). The rates of intra-hospital MACE (57.1% [50.5%;63.5%] vs (61.3% [54.3%;67.9%]), p=0.38) and major bleedings (18.9% [14.2%;24.6%] vs (18.6% [13.7%;24.6%], p=0.51) did not differ significantly in pre-treated patients compared to non-pre-treated ones.
Conclusion: In our cohort of patients with OHCA suspected of ACS, we did not observe an association between pre-hospital administration of an antiplatelet loading dose and outcomes.
Purpose: To evaluate efficacy and safety of pre-hospital administration of antiplatelet therapy in patients with OHCA directed to a cardiac catheterization laboratory (cath lab).
Method: Using the cath lab database of a tertiary cardiovascular center, we included consecutive patients referred for coronary angiography within 24 hours of OHCA for suspected ACS from January 2012 to January 2024. Pre-hospital antiplatelet treatment was defined as prescribing any antiplatelet therapy before cath lab admission (aspirin alone or combined with a P2Y12 inhibitor). The outcomes of interest were: 1) all-cause death at 30 days, 2) intra-hospital major adverse cardiovascular events (MACE), defined by the composite of all-cause death, myocardial infarction, or stroke, and 3) intra-hospital major bleedings (BARC ≥ 3). To help account for the non-randomized pre-hospital antiplatelet administration, an Inverse Probability Weighting (IPW) approach was used to compare outcomes between the two groups.
Result: Between January 2012 and January 2024, n = 411 patients with OHCA were referred to the cath lab, of whom 217 (52.8%) received a pre-treatment. Pre-hospital antiplatelet administration did not show a reduced risk of death from any cause at 30 days (56.7% [50.0%;63.1%] vs 59.8% [52.8%;66.4%], p=0.21) (Figure 1). The rates of intra-hospital MACE (57.1% [50.5%;63.5%] vs (61.3% [54.3%;67.9%]), p=0.38) and major bleedings (18.9% [14.2%;24.6%] vs (18.6% [13.7%;24.6%], p=0.51) did not differ significantly in pre-treated patients compared to non-pre-treated ones.
Conclusion: In our cohort of patients with OHCA suspected of ACS, we did not observe an association between pre-hospital administration of an antiplatelet loading dose and outcomes.
- Charleux, Pierre ( Sorbonne University - APHP , Paris , Texas , United States )
- Demoule, Alexandre ( Sorbonne University - APHP , Paris , Texas , United States )
- Combes, Alain ( Sorbonne University - APHP , Paris , Texas , United States )
- Silvain, Johanne ( Sorbonne University - APHP , Paris , Texas , United States )
- Montalescot, Gilles ( Sorbonne University - APHP , Paris , Texas , United States )
- Zeitouni, Michel ( Sorbonne University - APHP , Paris , Texas , United States )
- Chommeloux, Juliette ( Sorbonne University - APHP , Paris , Texas , United States )
- Elhadad, Anthony ( Sorbonne University - APHP , Paris , Texas , United States )
- Procopi, Niki ( Sorbonne University - APHP , Paris , Texas , United States )
- Guedeney, Paul ( Sorbonne University - APHP , Paris , Texas , United States )
- Martinez, Clelia ( Sorbonne University - APHP , Paris , Texas , United States )
- Rouanet, Stephanie ( StatEthic , Levallois Perret , France )
- Vicaut, Eric ( URC Lariboisière-St Louis , Paris , France )
- Kerneis, Mathieu ( Sorbonne University - APHP , Paris , Texas , United States )
Author Disclosures:
Pierre CHARLEUX: DO NOT have relevant financial relationships
| Alexandre Demoule: No Answer
| Alain COMBES: No Answer
| Johanne Silvain: DO NOT have relevant financial relationships
| Gilles Montalescot: DO have relevant financial relationships
;
Other (please indicate in the box next to the company name):Abbott : research or educational grant to the institution and consulting or lecture fees:Active (exists now)
; Other (please indicate in the box next to the company name):Terumo : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):SMT : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):Pfizer:Active (exists now)
; Other (please indicate in the box next to the company name):Novo Nordisk : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):Lilly:Past (completed)
; Other (please indicate in the box next to the company name):Idorsia:Active (exists now)
; Other (please indicate in the box next to the company name):Hexacath:Past (completed)
; Other (please indicate in the box next to the company name):CSL Behring : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):Celecor : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):Boehringer Ingelheim : research or educational grant to the institution and consulting or lecture fees:Active (exists now)
; Other (please indicate in the box next to the company name):BMS : research or educational grant to the institution and consulting or lecture fees:Active (exists now)
; Other (please indicate in the box next to the company name):Bayer : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):AstraZeneca : research or educational grant to the institution and consulting or lecture fees:Past (completed)
; Other (please indicate in the box next to the company name):Amgen : research or educational grant to the institution and consulting or lecture fees:Past (completed)
| Michel Zeitouni: DO NOT have relevant financial relationships
| Juliette Chommeloux: No Answer
| Anthony Elhadad: DO NOT have relevant financial relationships
| Niki PROCOPI: No Answer
| Paul Guedeney: No Answer
| Clelia Martinez: No Answer
| Stephanie Rouanet: DO NOT have relevant financial relationships
| Eric VICAUT: No Answer
| Mathieu Kerneis: No Answer
Meeting Info:
Session Info:
More abstracts on this topic:
A Personal Risk Assessment Device in Patients with Chest Pain
Shvilkin Alexei, Zlatic Natasa, Atanasoski Vladimir, Grujovic Zdolsek Sanja, Popovic Maneski Lana, Miletic Marjan, Vukcevic Vladan
A Polypill Strategy for Lipid Lowering and Anti-Platelet Therapy After Acute Coronary Syndrome: A Pilot Randomized Controlled TrialKeshvani Neil, Wang Thomas, Pandey Ambarish, Coellar Juan David, Rizvi Syed Kazim, Jain Anand, Bustillo-rubio M. Karina, Segar Matthew, Lokesh Nidhish, Miller James, Yates Sean
More abstracts from these authors:
Your ECPR truck is cool ... but ECPR is not ready for prime time
Combes Alain, Soeholm Helle
Cardiovascular And Obstetrical Outcomes In Women With Premature Coronary Artery DiseaseMikou Meryem, Zeitouni Michel, Procopi Niki, Charleux Pierre, Rahoual Ghilas, Kerneis Mathieu, Silvain Johanne, Legrand Lise, Nizard Jacky, Montalescot Gilles
You have to be authorized to contact abstract author. Please, Login
Not Available