Final ID: Sa4179
Study of Angiogenic Cell Therapy for Progressive Pulmonary Hypertension: Intervention with Repeat dosing of eNOS-enhanced EPCs: the SAPPHIRE Trial
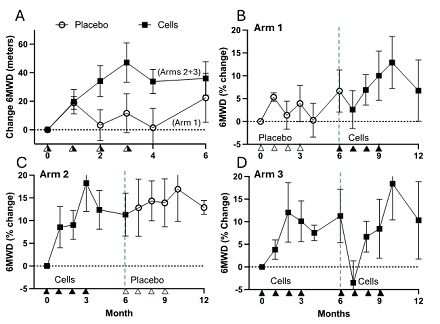
Abstract Body (Do not enter title and authors here): In pulmonary arterial hypertension (PAH), widespread pruning of the lung distal arterial bed leads to increased vascular resistance, right heart failure and death. Currently, there are no available therapies designed to revascularize the lung. We assessed the effects of autologous, angiogenic endothelial progenitor cells (EPCs) transfected with endothelial NO synthase (eNOS) in PAH patients receiving available standard-of-care therapies in a 12-month randomized, controlled trial (RCT; NCT03001414). Patients were randomized to receive a course of 4 monthly infusions over the first 6 months consisting of placebo (Arm 1) or eNOS-EPCs (20M cells; Arms 2 and 3) at 0, 1, 2 and 3 months, for a total 80M cells. After 6 months, Arm 1 crossed over to cell infusions, Arm 2 to placebo, whereas Arm 3 received a second course of eNOS-EPCs (total 160M cells). The primary endpoint was change in 6MWD (baseline to 6 months) between patients receiving eNOS-EPCs vs. placebo. However, due to the COVID-19 pandemic the trial was stopped after only 12 of the planned 45 patients were enrolled (5 placebo, 7 cells). While small sample size precluded appropriately powered analyses, a qualitative difference was seen between the eNOS-EPC and placebo groups over the first 6 months (Figure), with a 2-fold greater proportion of patients achieving a 35-meter increase in 6MWD in the cell therapy group (40% vs. 20%, resp). As well, these improvements coincided with cell delivery in all 3 Arms, with a sustained increase in 6MWD over 12 months after a single course of cells in the first 6 months (Arm 2). Treatment with eNOS-EPCs was well tolerated; however, an isolated decrease in 6MWD was apparent 1 month after the first infusion of the second course of cell therapy in Arm 3 patients, with full recovery by the end of the course. This raises the possibility of an immune response to rechallenging patients with this autologous cell product, despite the absence of any clinical indicators, which will be explored by analysis of immune biomarkers. Therefore, the results of the SAPPHIRE trial suggest possible clinical benefit of a single course of 4 infusions of eNOS-EPCs in PAH patients receiving cell therapy and support the performance of a larger RCT.
More abstracts on this topic:
The Angiogenic Potential of Dipeptidyl Peptidase 4 Inhibition in a Large Animal Model of Coronary Artery Disease in the Setting of Metabolic Syndrome
Muir Kelsey, Harris Dwight, Stone Christopher, Kanuparthy Meghamsh, Hamze Jad, Feng Jun, Sellke Frank
CPSF7-Mediated Alternative Polyadenylation of CCNE2 Enhances the Regenerative Capacity of Human iPSC-Derived Cardiomyocytes for Myocardial Infarction TherapyZhou Huatao, Tang Weijie, Zheng Zilong, Yang Jinfu, Fan Chengming