Final ID: MDP79
Functional evidence of regulation of the NaV1.5 channel through homomeric interaction of its α-subunits in the cellular membrane
Abstract Body (Do not enter title and authors here): Recent structural findings reveled potential interaction between NaV1.5 in the plasma membrane. However, functional effects of this interaction on Na+ current (INa) remain elusive.
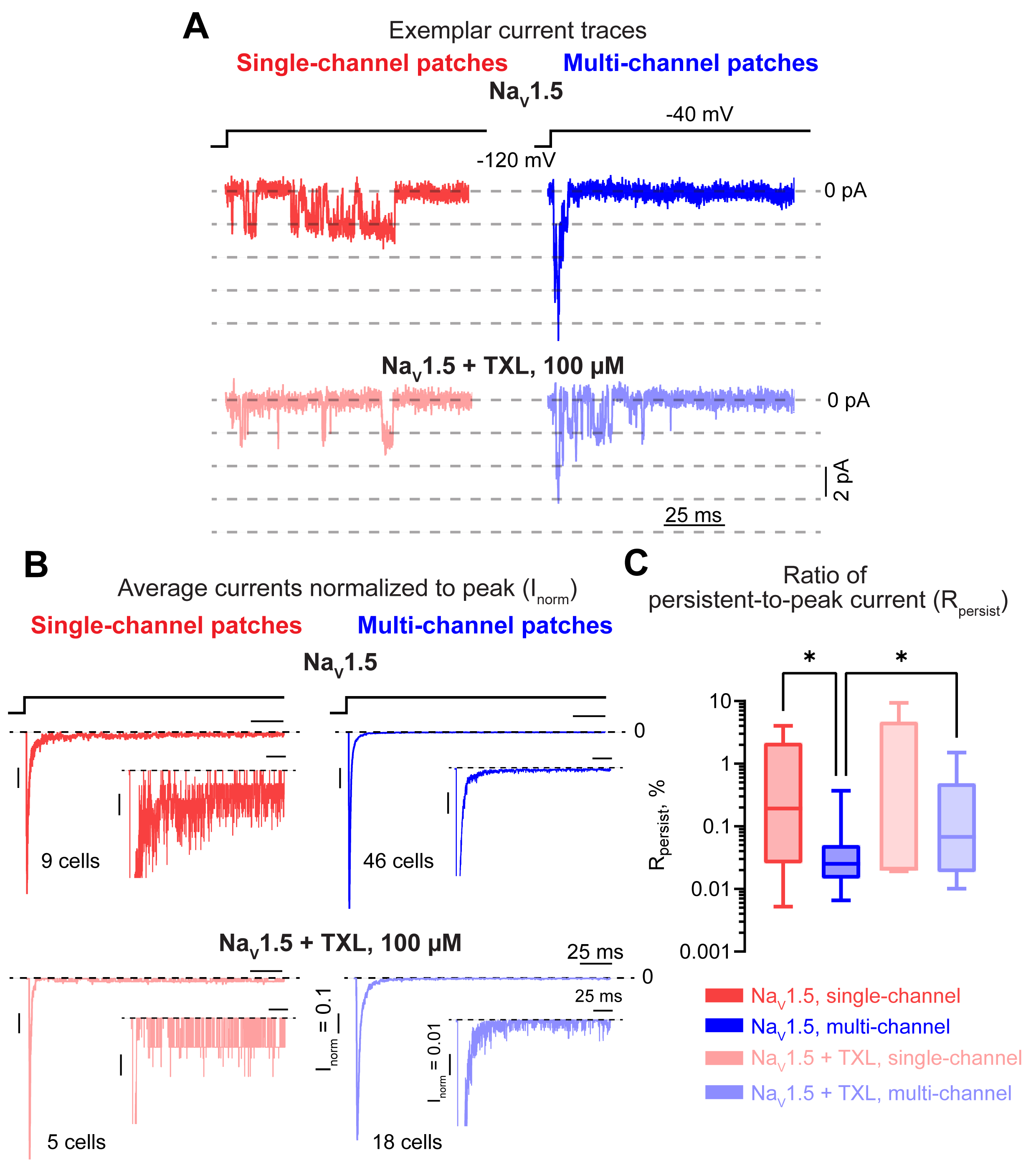
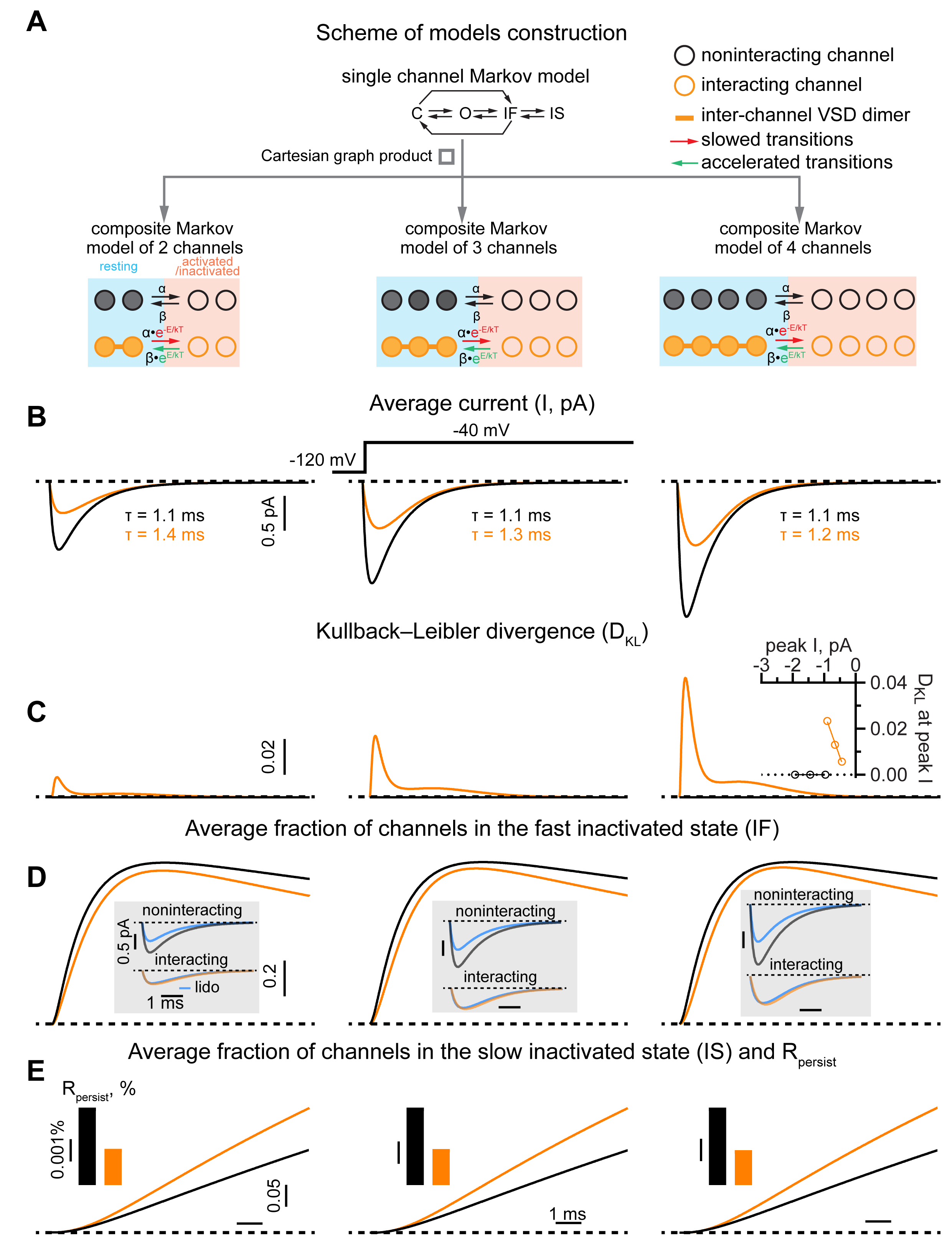
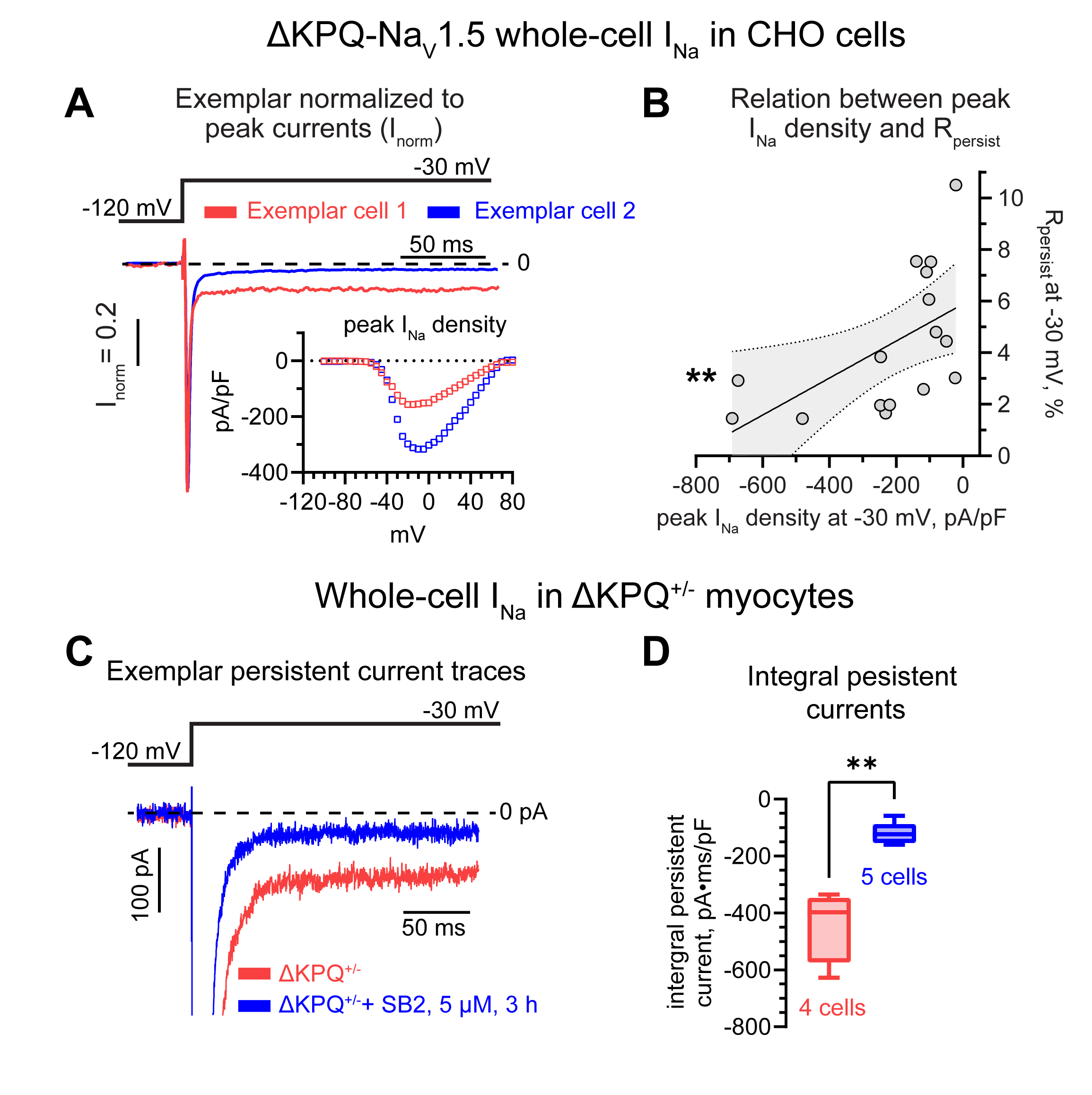
Cell-attached patch-clamp in Chinese hamster ovary (CHO) cells stably expressing NaV1.5 revealed a significant increase in the ratio of persistent-to-peak INa (Rpersist) in single- vs. multi-channel patches. These data suggest that reduced NaV1.5 surface density impacts Rpersist. Indeed, reduction of NaV1.5 membrane density by paclitaxel (TXL, 100 µM, 2 hours) significantly increased Rpersist of multi- but not single-channel patches relative to untreated NaV1.5 expressing cells (Fig. 1). This was further supported by a divergence in peak INa predicted by a single-channel Markov model based on single-channel activity relative to experimental multi-channel recordings. To overcome the limitation of this classical model, we implemented a novel Markov model which accounts for channel interaction. Importantly, this approach not only replicated our experimental findings but also predicted a reduction of a fraction of channels in the fast inactivated state and consequently an acceleration of recovery from fast inactivation in multi-channel recordings (Fig. 2). Consistent with this prediction, experimental application of lidocaine (50 µM), an agent which blocks NaV1.5 during fast inactivation, exhibited reduced inhibitory effect in multi- vs. single-channel patches. Confirming the dependence of Rpersist on NaV1.5 membrane density, whole-cell patch clamp recordings in CHO cells transiently transfected with the inactivation-deficient ΔKPQ-NaV1.5 mutant reveled a significant negative correlation between the magnitude of whole-cell peak INa density and Rpersist. Notably, increasing NaV1.5 cluster density with SB216763 (5 µM) in cardiomyocytes isolated from mice harboring this mutation (ΔKPQ+/-) significantly reduced whole-cell Rpersist relative to untreated myocytes (Fig. 3).
Our study suggests that NaV1.5 homomeric interaction may impact NaV1.5 kinetics and can serve as a therapeutic target for arrhythmia prevention.
Cell-attached patch-clamp in Chinese hamster ovary (CHO) cells stably expressing NaV1.5 revealed a significant increase in the ratio of persistent-to-peak INa (Rpersist) in single- vs. multi-channel patches. These data suggest that reduced NaV1.5 surface density impacts Rpersist. Indeed, reduction of NaV1.5 membrane density by paclitaxel (TXL, 100 µM, 2 hours) significantly increased Rpersist of multi- but not single-channel patches relative to untreated NaV1.5 expressing cells (Fig. 1). This was further supported by a divergence in peak INa predicted by a single-channel Markov model based on single-channel activity relative to experimental multi-channel recordings. To overcome the limitation of this classical model, we implemented a novel Markov model which accounts for channel interaction. Importantly, this approach not only replicated our experimental findings but also predicted a reduction of a fraction of channels in the fast inactivated state and consequently an acceleration of recovery from fast inactivation in multi-channel recordings (Fig. 2). Consistent with this prediction, experimental application of lidocaine (50 µM), an agent which blocks NaV1.5 during fast inactivation, exhibited reduced inhibitory effect in multi- vs. single-channel patches. Confirming the dependence of Rpersist on NaV1.5 membrane density, whole-cell patch clamp recordings in CHO cells transiently transfected with the inactivation-deficient ΔKPQ-NaV1.5 mutant reveled a significant negative correlation between the magnitude of whole-cell peak INa density and Rpersist. Notably, increasing NaV1.5 cluster density with SB216763 (5 µM) in cardiomyocytes isolated from mice harboring this mutation (ΔKPQ+/-) significantly reduced whole-cell Rpersist relative to untreated myocytes (Fig. 3).
Our study suggests that NaV1.5 homomeric interaction may impact NaV1.5 kinetics and can serve as a therapeutic target for arrhythmia prevention.
More abstracts on this topic:
A High Salt Diet Drives Kidney Microvascular Dysfunction Through a Plasma-Derived Factor that Increases Mitochondrial Reactive Oxygen Species
Reynolds Lance, Guan Zhengrong, Pollock David, Pollock Jennifer
An Artificial Intelligence Machine Learning Algorithm Approach Using Segmental ECG Analysis to Distinguish Long QT Syndrome from Acquired QT ProlongationBos Johan, Liu Kan, Attia Zachi, Noseworthy Peter, Friedman Paul, Ackerman Michael