Final ID: MDP355
DOT1L mediates diabetic vascular remodeling by promoting insulin-induced phenotypic conversion of VSMCs through the NOTCH1-related pathway
Abstract Body (Do not enter title and authors here): Introduction: The transformation of vascular smooth muscle cells (VSMCs) from contractile to synthetic is an important step in diabetic vascular remodeling. DOT1L, the only lysine methyltransferase at the H3K79me locus, has not been fully elucidated in diabetic vascular remodeling, and DOT1L-based methods may be critical in discovering phenotypic transformation of VSMCs to improve clinical outcomes in patients with diabetic cardiovascular disease.
Hypothesis: Whether DOT1L promotes the phenotypic transformation of VSMCs and further affects diabetic vascular remodeling.
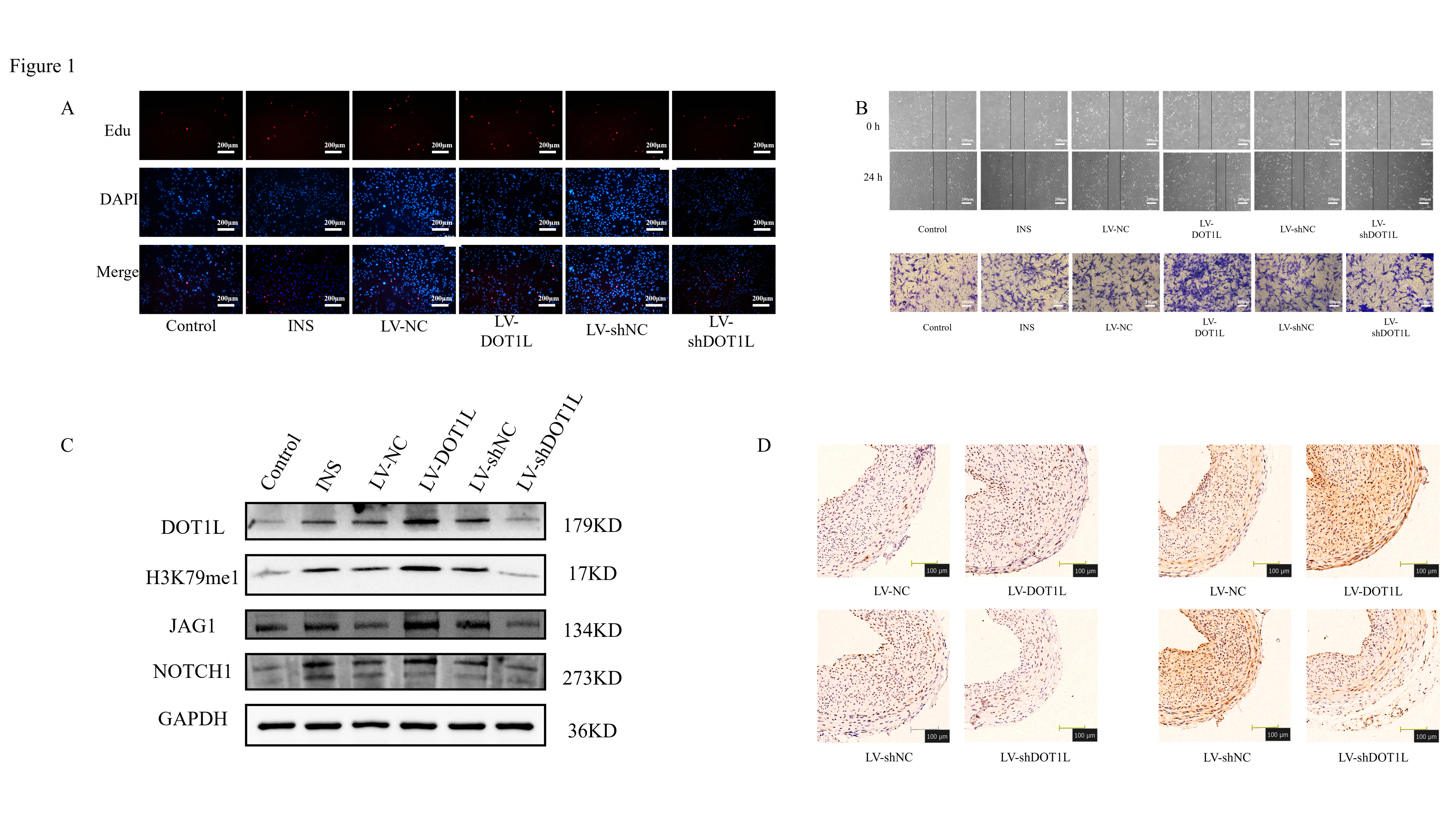
Methods: Primary VSMCs were extracted from the thoracic aorta of healthy, SPF-free male rats. DOT1L was up-regulated or down-regulated in vitro by lentivirus infection with VSMCs, followed by an insulin stimulation. VSMCs phenotype was assessed by EdU, flow cytometry, scratch assay, Transwell chamber assay, western blot; In addition, the mechanism of DOT1L on insulin-induced phenotypic transformation of VSMCs was explored. SD rats were fed with high glucose and high fat for 4 weeks, and were given a single intraperitoneal injection of low-dose (30 mg) STZ to establish a diabetes model. After 2 weeks of continuous high-glucose and high-fat feeding, a carotid balloon injury model was established, and then lentivirus was injected into the injured artery segment to overexpress or knock down DOT1L in vivo, and the role and mechanism of diabetic VSMCs phenotype were evaluated by immunohistochemistry and western blot.
Results: After 24 hours of stimulation with 100 nmol/L insulin, the expressions of VSMCs synthesized phenotype OPN, proliferative phenotype PCNA, migration phenotype MMP2 and MMP9 increased, the expression of α-SMA decreased, and proliferation and migration occurred. Down-regulation of DOT1L can inhibit the conversion of insulin-induced VSMCs from contractile to synthetic and improve diabetic vascular remodeling. Mechanistically, overexpression of DOT1L promotes the activation of JAG1/NOTCH1 signaling pathway, promotes the expression of synthetic phenotypic proteins, and inhibits the expression of contractile phenotypic proteins, which ultimately promotes the transformation of VSMCs from contractile to synthetic in diabetic environment.
Conclusions: Down-regulation of DOT1L can inhibit the activation of JAG1/NOTCH1 signaling pathway, thereby inhibiting the phenotypic transformation of VSMCs and improving diabetic vascular remodeling.
Hypothesis: Whether DOT1L promotes the phenotypic transformation of VSMCs and further affects diabetic vascular remodeling.
Methods: Primary VSMCs were extracted from the thoracic aorta of healthy, SPF-free male rats. DOT1L was up-regulated or down-regulated in vitro by lentivirus infection with VSMCs, followed by an insulin stimulation. VSMCs phenotype was assessed by EdU, flow cytometry, scratch assay, Transwell chamber assay, western blot; In addition, the mechanism of DOT1L on insulin-induced phenotypic transformation of VSMCs was explored. SD rats were fed with high glucose and high fat for 4 weeks, and were given a single intraperitoneal injection of low-dose (30 mg) STZ to establish a diabetes model. After 2 weeks of continuous high-glucose and high-fat feeding, a carotid balloon injury model was established, and then lentivirus was injected into the injured artery segment to overexpress or knock down DOT1L in vivo, and the role and mechanism of diabetic VSMCs phenotype were evaluated by immunohistochemistry and western blot.
Results: After 24 hours of stimulation with 100 nmol/L insulin, the expressions of VSMCs synthesized phenotype OPN, proliferative phenotype PCNA, migration phenotype MMP2 and MMP9 increased, the expression of α-SMA decreased, and proliferation and migration occurred. Down-regulation of DOT1L can inhibit the conversion of insulin-induced VSMCs from contractile to synthetic and improve diabetic vascular remodeling. Mechanistically, overexpression of DOT1L promotes the activation of JAG1/NOTCH1 signaling pathway, promotes the expression of synthetic phenotypic proteins, and inhibits the expression of contractile phenotypic proteins, which ultimately promotes the transformation of VSMCs from contractile to synthetic in diabetic environment.
Conclusions: Down-regulation of DOT1L can inhibit the activation of JAG1/NOTCH1 signaling pathway, thereby inhibiting the phenotypic transformation of VSMCs and improving diabetic vascular remodeling.
More abstracts on this topic:
A Clinical Trial of Healthy Food Subsidies and Behavioral Interventions to Increase Fruit and Vegetable Purchasing in an Online Store
Hua Sophia, Klaiman Tamar, Dixon Erica, Volpp Kevin, Putt Mary, Coratti Samantha, White Jenna, Hossain Mohammad, Posner Hannah, Wang Erkuan, Zhu Jingsan, John Aileen
Antihypertensive Medication Prescription Patterns at Baseline in the LINKED-HEARTS Program: A Comparative Analysis with American Heart Association GuidelinesAdomako Nana Ofori, Chen Yuling, Demarco Samantha, Chepkorir Joyline, Owusu Nti Kezia, Slone Sarah, Commodore-mensah Yvonne, Himmelfarb Cheryl