Final ID: Sa1082
From Atrial Fibrillation to Rare Genetic Disorder: The Unexpected Diagnosis of Birt-Hogg-Dubé Syndrome
Abstract Body (Do not enter title and authors here): Introduction
Birt-Hogg-Dubé syndrome (BHDS) is a rare autosomal-dominant disorder due to mutations in the FLCN gene. As an AMPK-mTOR interacting molecule, this loss-of-function mutation impacts energy homeostasis leading to uninhibited cell growth. While studies have linked this genetic mutation to severe cardiac hypertrophy, a connection to persistent atrial fibrillation (AF) is unestablished. We present a case in which an individual's initial symptoms of AF ultimately led to a diagnosis of BHDS.
Case Summary
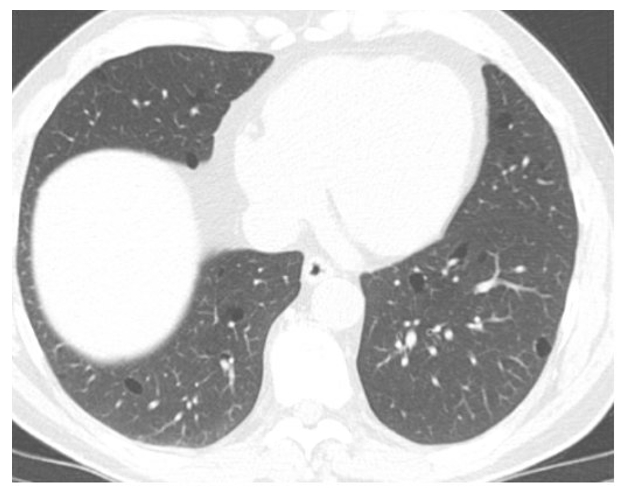
A 56-year-old male with a history of “holiday heart syndrome” presented for an AF consultation. Despite alcohol cessation, he continued to have paroxysmal AF with severe fatigue and palpitations. After three unsuccessful cardioversions and refractory episodes despite Dronedarone therapy, he was scheduled for an ablation. A pre-procedural transesophageal echocardiogram showed a left ventricular (LV) ejection fraction of 55%, mild concentric LV hypertrophy, and mild left atrial enlargement. These enlargements were new compared with imaging one year prior. A cardiac gated CT detailed the pulmonary vein structure and incidentally revealed bilateral cystic lung changes, leading to a pulmonology referral. Despite an initial successful ablation targeting the pulmonary vein, his symptoms returned within days. He was loaded with amiodarone. Monitoring on his apple watch showed a high burden of AF. He underwent a repeat ablation to achieve complete pulmonary vein isolation with improvement in his symptoms. Further pulmonary evaluation highlighted a family history of spontaneous pneumothorax and a dermatology consultation then confirmed BHDS via fibrofolliculoma biopsy.
Discussion
This case underscores the importance of comprehensive evaluations, revealing a rare genetic disorder from an initial AF assessment. While no definitive link exists between BHDS and cardiomyopathy, we aim to understand possible connections between this rare condition and its systemic cardiovascular impacts. We propose that upregulation of mTORC1 activity and attenuation of AMPK from the loss of function of FLCN lead to uncontrolled cell division and left ventricular hypertrophy. This hypertrophy then results in left atrial dilation, contributing to the development and persistence of AF. Given the rarity of this condition, more research is needed on symptom onset and systemic linkages. Swift diagnosis from incidental findings are crucial for managing symptoms and the underlying disease.
Birt-Hogg-Dubé syndrome (BHDS) is a rare autosomal-dominant disorder due to mutations in the FLCN gene. As an AMPK-mTOR interacting molecule, this loss-of-function mutation impacts energy homeostasis leading to uninhibited cell growth. While studies have linked this genetic mutation to severe cardiac hypertrophy, a connection to persistent atrial fibrillation (AF) is unestablished. We present a case in which an individual's initial symptoms of AF ultimately led to a diagnosis of BHDS.
Case Summary
A 56-year-old male with a history of “holiday heart syndrome” presented for an AF consultation. Despite alcohol cessation, he continued to have paroxysmal AF with severe fatigue and palpitations. After three unsuccessful cardioversions and refractory episodes despite Dronedarone therapy, he was scheduled for an ablation. A pre-procedural transesophageal echocardiogram showed a left ventricular (LV) ejection fraction of 55%, mild concentric LV hypertrophy, and mild left atrial enlargement. These enlargements were new compared with imaging one year prior. A cardiac gated CT detailed the pulmonary vein structure and incidentally revealed bilateral cystic lung changes, leading to a pulmonology referral. Despite an initial successful ablation targeting the pulmonary vein, his symptoms returned within days. He was loaded with amiodarone. Monitoring on his apple watch showed a high burden of AF. He underwent a repeat ablation to achieve complete pulmonary vein isolation with improvement in his symptoms. Further pulmonary evaluation highlighted a family history of spontaneous pneumothorax and a dermatology consultation then confirmed BHDS via fibrofolliculoma biopsy.
Discussion
This case underscores the importance of comprehensive evaluations, revealing a rare genetic disorder from an initial AF assessment. While no definitive link exists between BHDS and cardiomyopathy, we aim to understand possible connections between this rare condition and its systemic cardiovascular impacts. We propose that upregulation of mTORC1 activity and attenuation of AMPK from the loss of function of FLCN lead to uncontrolled cell division and left ventricular hypertrophy. This hypertrophy then results in left atrial dilation, contributing to the development and persistence of AF. Given the rarity of this condition, more research is needed on symptom onset and systemic linkages. Swift diagnosis from incidental findings are crucial for managing symptoms and the underlying disease.
More abstracts on this topic:
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine model
Lee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail
A novel LMNA mutant (R225X) leads to cardiac conduction disordersLi Tingting, Chelu Mihail, Wang Xiaolei, Song Jia, Li Luge, Gutierrez Yaqueline, Shinohara Anna, Whitfield William, Adeleye Adeniyi, Li Na