Final ID: MDP1172
Artificial Intelligence Can Match Atrial Fibrillation Electrograms to Action Potential Recordings in Patients
Abstract Body (Do not enter title and authors here): Background:
In patients with atrial fibrillation (AF), pulmonary vein isolation (PVI) improves morbidity and mortality compared to anti-arrhythmic medications, yet is suboptimal in >30-40% of patients typically with persistent AF. Several promising mapping approaches are emerging for such patients, but results are inconsistent. One challenge is to reconcile differences between clinical mapping of electrograms and optical mapping, that detects local tissue action potentials.
Hypothesis:
We hypothesized that artificial intelligence (AI) algorithms applied to AF electrograms recorded from clinical catheters can infer onsets of action potentials, referenced to clinical MAP recordings.
Methods:
In N=303 AF patients at ablation (68.2±8.0 years, 17.8% females, 72.9% non-paroxysmal AF), we isolated a development cohort (N=229) and a test cohort (N=74). In the development cohort we trained a multistage AI-system to identify cycle-to-cycle activations in AF electrograms recorded from various clinical catheters. In the separate test cohort, we assessed the accuracy of the AI algorithm relative to MAP onsets, recorded at adjacent sites with dedicated catheters (N=20, MedFact, GmbH). We related accuracy to signal quality, defined from the homogeneity of distribution of activation times.
Results:
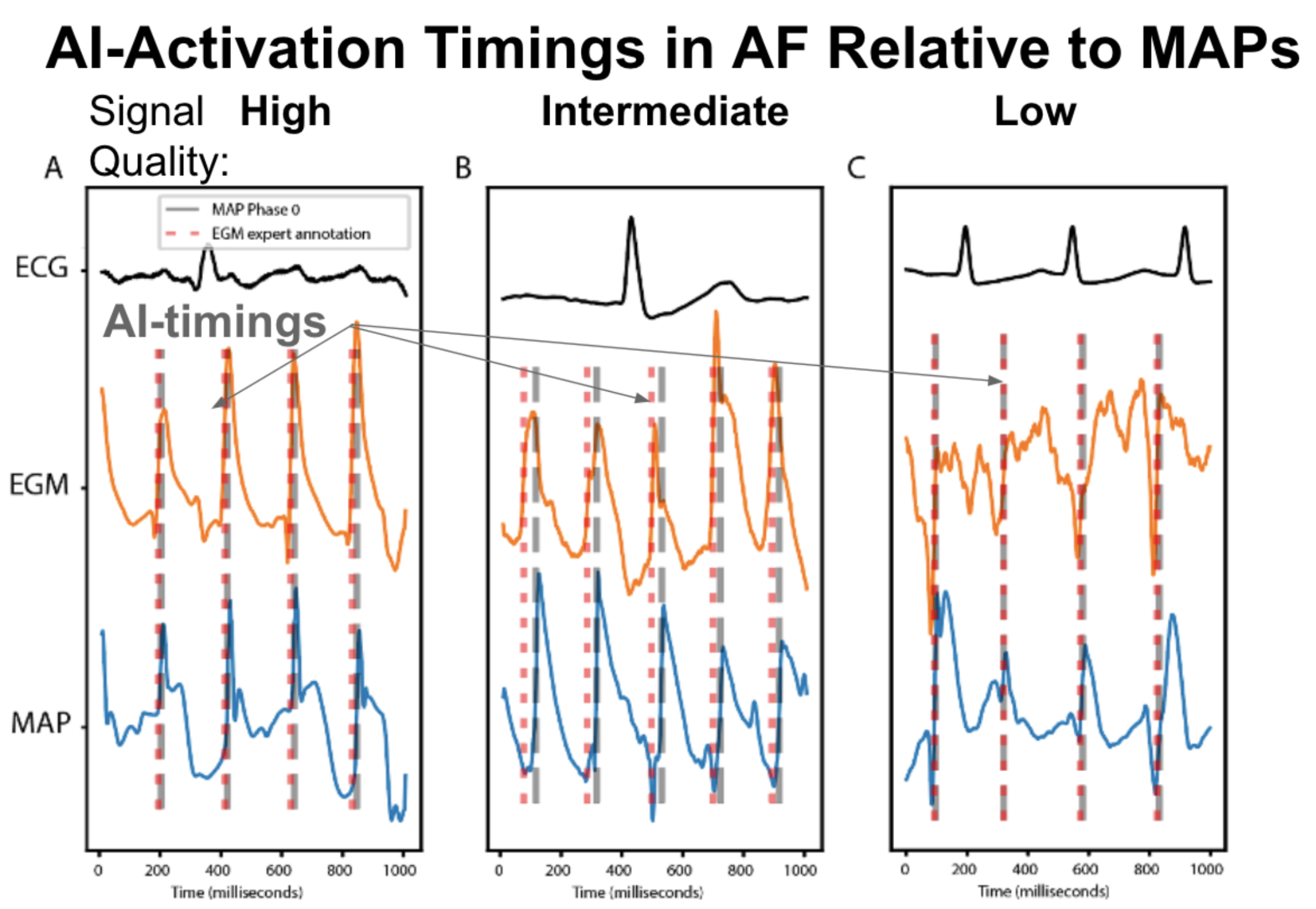
Figures shows unipolar AF EGMs, MAPs and AI annotation. A. Good agreement between AI timing, and MAP onsets (15 ms) for signals of high quality in a 67 year old woman. B. Good agreement between AI timings and MAP onsets (42 ms) with delay reflecting spatial separation between EGM and MAP catheters in a 72 year old woman. C. Good agreement despite difficulty in expert annotation of EGM in cases of intermediate to low signal quality in a 67 year old man. In summary, the AI system provided an average F1-score for AF timings of 0.83 relative to experts (p<0.01), varying from 0.72 to 0.95 for low to high signal quality (p<0.01). A similar trend was found for AI accuracy vs. MAP onsets (p<.05)
Conclusions:
In this large registry, a novel AI-based approach accurately identified AF activation relative to tissue action potential onsets, referenced to expert labels. AI may improve characterization of AF activity within the atria, and could be a foundation for future mapping and phenotyping.
In patients with atrial fibrillation (AF), pulmonary vein isolation (PVI) improves morbidity and mortality compared to anti-arrhythmic medications, yet is suboptimal in >30-40% of patients typically with persistent AF. Several promising mapping approaches are emerging for such patients, but results are inconsistent. One challenge is to reconcile differences between clinical mapping of electrograms and optical mapping, that detects local tissue action potentials.
Hypothesis:
We hypothesized that artificial intelligence (AI) algorithms applied to AF electrograms recorded from clinical catheters can infer onsets of action potentials, referenced to clinical MAP recordings.
Methods:
In N=303 AF patients at ablation (68.2±8.0 years, 17.8% females, 72.9% non-paroxysmal AF), we isolated a development cohort (N=229) and a test cohort (N=74). In the development cohort we trained a multistage AI-system to identify cycle-to-cycle activations in AF electrograms recorded from various clinical catheters. In the separate test cohort, we assessed the accuracy of the AI algorithm relative to MAP onsets, recorded at adjacent sites with dedicated catheters (N=20, MedFact, GmbH). We related accuracy to signal quality, defined from the homogeneity of distribution of activation times.
Results:
Figures shows unipolar AF EGMs, MAPs and AI annotation. A. Good agreement between AI timing, and MAP onsets (15 ms) for signals of high quality in a 67 year old woman. B. Good agreement between AI timings and MAP onsets (42 ms) with delay reflecting spatial separation between EGM and MAP catheters in a 72 year old woman. C. Good agreement despite difficulty in expert annotation of EGM in cases of intermediate to low signal quality in a 67 year old man. In summary, the AI system provided an average F1-score for AF timings of 0.83 relative to experts (p<0.01), varying from 0.72 to 0.95 for low to high signal quality (p<0.01). A similar trend was found for AI accuracy vs. MAP onsets (p<.05)
Conclusions:
In this large registry, a novel AI-based approach accurately identified AF activation relative to tissue action potential onsets, referenced to expert labels. AI may improve characterization of AF activity within the atria, and could be a foundation for future mapping and phenotyping.
More abstracts on this topic:
A Deep Learning Digital Biomarker for Mitral Valve Prolapse using Echocardiogram Videos
Al-alusi Mostafa, Khurshid Shaan, Sanborn Danita, Picard Michael, Ho Jennifer, Maddah Mahnaz, Ellinor Patrick, Lau Emily, Small Aeron, Reeder Christopher, Shnitzer Dery Tal, Andrews Carl, Kany Shinwan, Ramo Joel, Haimovich Julian
Comparing 1-Year and 3-Year Outcomes of Atrial Fibrillation AblationSrivastava Viren, Clopton Paul, Brodt Chad, Narayan Sanjiv, Deb Brototo, Chang Hui Ju, Ganesan Prasanth, Brennan Kelly, Feng Ruibin, Rogers Albert, Baykaner Tina, Wang Paul