Final ID: Su2104
Redefining atrial fibrillation treatment success: Institutional results of atrial fibrillation care using patient-defined pragmatic goals
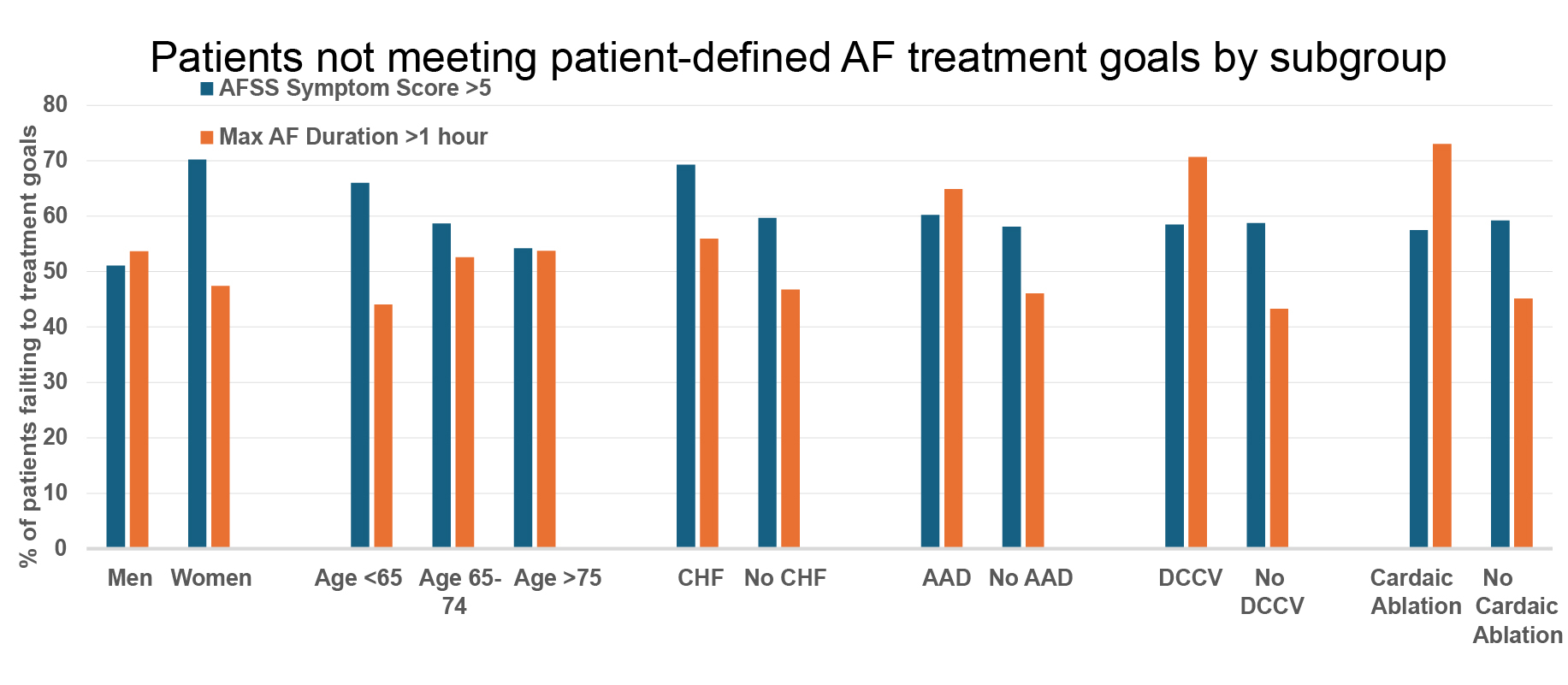
Methods: Using an institutional retrospective cohort of AF patients with either AFSS scores (53.8%) and/or ambulatory ECG (AECG) data (59.4%), we describe the proportion of patients failing to achieve an AFSS ≤ 5 or AF episodes ≤ 1 hour (Figure 1).
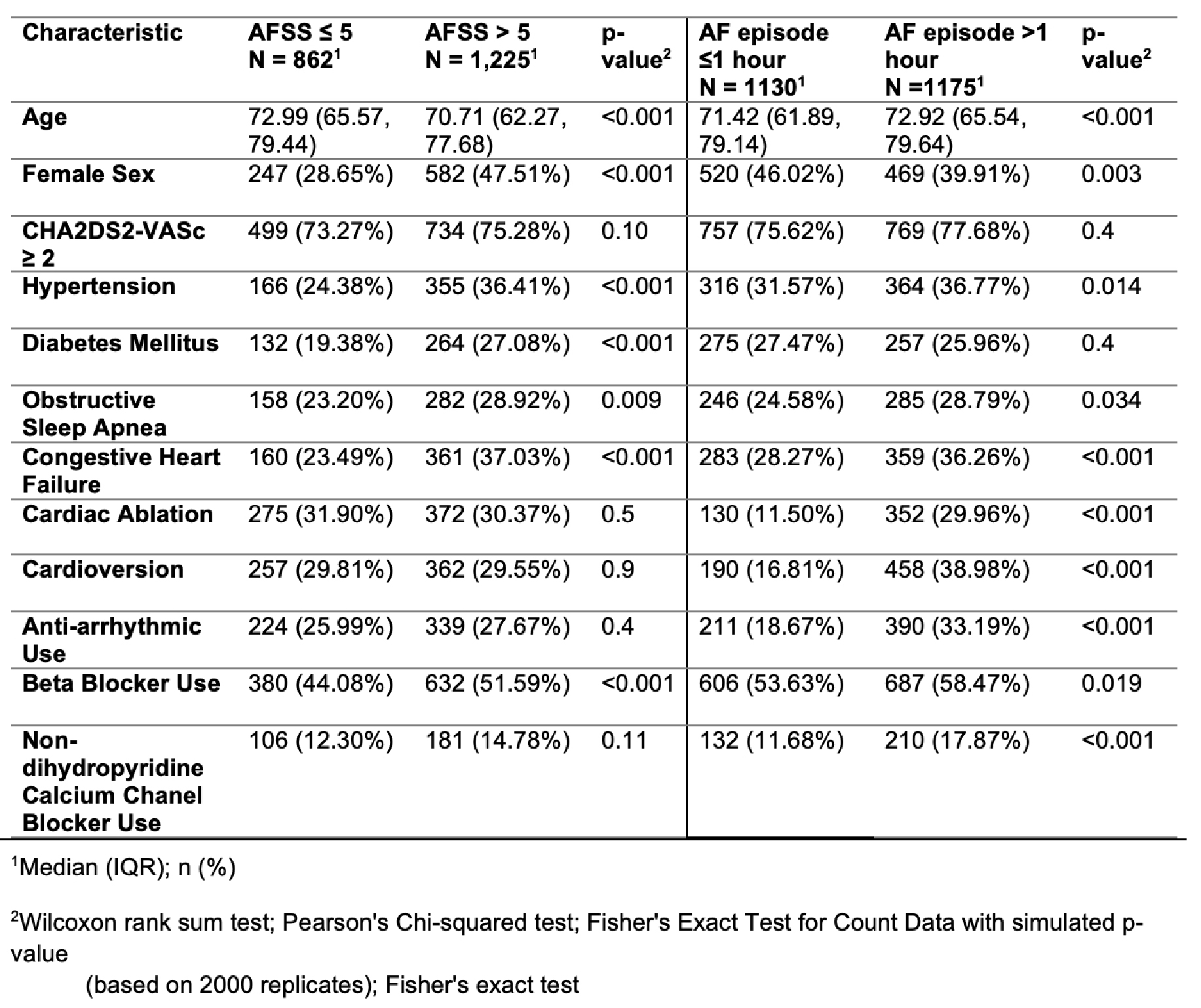
Results: Between 2019 and May 2024, 2087 patients with an AFSS symptom score, and 2305 with AECG monitoring were included, of whom 862 (41%) had AFSS scores ≤5 and 1130 (49%) had AF episodes ≤1 hour. Compared to patients with AFSS ≤ 5, 1225 patients (59%) with AFSS > 5 were younger, more likely to be female, and have more comorbid conditions (hypertension, diabetes mellitus, obstructive sleep apnea, and heart failure) without significant differences in treatment, including rhythm control strategies (Table 1). Compared to patients with AF episodes ≤ 1 hour, 1175 patients (51%) with AF episodes > 1 hour were more likely to have received prior ablation, prior cardioversion, and anti-arrhythmic medications.
Conclusion: Less than half of AF patients in clinical practice have AF symptom scores or arrhythmia burdens meeting patient-defined goals, with significant differences in both success measures by patient characteristics. Pursuing goal-oriented treatment in AF research and care may help clinicians identify uncontrolled patients, align treatment to patient priorities, and direct future medical intervention at an individual level.
- Kolomaya, Alexander ( University of Utah , Salt Lake City , Utah , United States )
- Redd, Andrew ( University of Utah , Salt Lake City , Utah , United States )
- Spertus, John ( Saint Lukes Mid America Heart Inst , Kansas City , Missouri , United States )
- Zhang, Yue ( University of Utah , Salt Lake City , Utah , United States )
- Steinberg, Benjamin ( University of Utah , Salt Lake City , Utah , United States )
- Zenger, Brian ( Washington University in St Louis , St. Louis , Missouri , United States )
- Torre, Michael ( University of Utah , Salt Lake City , Utah , United States )
- Lyons, Ann ( University of Utah , Salt Lake City , Utah , United States )
- Bunch, Thomas ( University of Utah , Salt Lake City , Utah , United States )
- Hess, Rachel ( University of Utah , Salt Lake City , Utah , United States )
- Piccini, Jonathan ( DUKE UNIVERSITY MEDICAL CENTER , Durham , North Carolina , United States )
- Lobban, Trudie ( Arrhythmia Alliance , Chipping Norton , United Kingdom )
- Miller, Morgan ( University of Utah , Salt Lake City , Utah , United States )
Meeting Info:
Session Info:
Atrial Fibrillation in Select Populations: Insights Into Management and Risk Stratification
Sunday, 11/17/2024 , 03:15PM - 04:15PM
Abstract Poster Session
More abstracts on this topic:
Qureshi Natasha, Vashistha Kirtivardhan, Ali Syed, Omar Ali, Kanei Yumiko
Adults with Arrhythmias and Cardiomyopathy Have Lower WHO-QOL Health Satisfaction and Different Wellness Intervention PreferencesSandau Kristin, Mathiason Michelle, Bai Ling, Conley Samantha
More abstracts from these authors:
Kolomaya Alexander, Zenger Brian, Ranjan Ravi, Bunch Thomas, Steinberg Benjamin
Temporal Trends of Contributing Cardiovascular Mechanisms of Mortality in Patients with Atrial Fibrillation Stratified by Age and SexGoodwin Ashley, Steinberg Benjamin, Bunch Thomas, Dickey Jacqueline, Endo Bobby, Chen Arthur, Engels Marc, Konstantinidis Klitos, Lehmann H. Immo, Packer Douglas, Ranjan Ravi