Final ID: Mo1004
Prevalence and Clinical Characteristics of US Patients with Systemic Inflammation and Atherosclerotic Cardiovascular Disease With or Without Chronic Kidney Disease
Abstract Body (Do not enter title and authors here): Background: High-sensitivity C-reactive protein (hsCRP) is a well-established biomarker of inflammation. Systemic inflammation is a recognized risk factor for atherosclerotic cardiovascular disease (ASCVD); however, the prevalence of systemic inflammation (SI) and the associated patient characteristics remain unknown.
Aim: To define the prevalence and clinical characteristics of patients with SI and ASCVD with or without chronic kidney disease (CKD).
Methods: A retrospective cross-sectional analysis of US adults (aged ≥18 years) with any diagnoses of ASCVD evaluated with a hsCRP test using the Optum® de-identified electronic health record dataset between 2017 and 2021. SI was defined as an hsCRP level of 2–10 mg/L. The prevalence of SI was evaluated by calendar year, stratified to three groups by presence of ASCVD, ASCVD with any stage of CKD, and ASCVD with stage 3 or 4 CKD. Concomitant comorbidities and medications used were assessed and stratified by CKD severity.
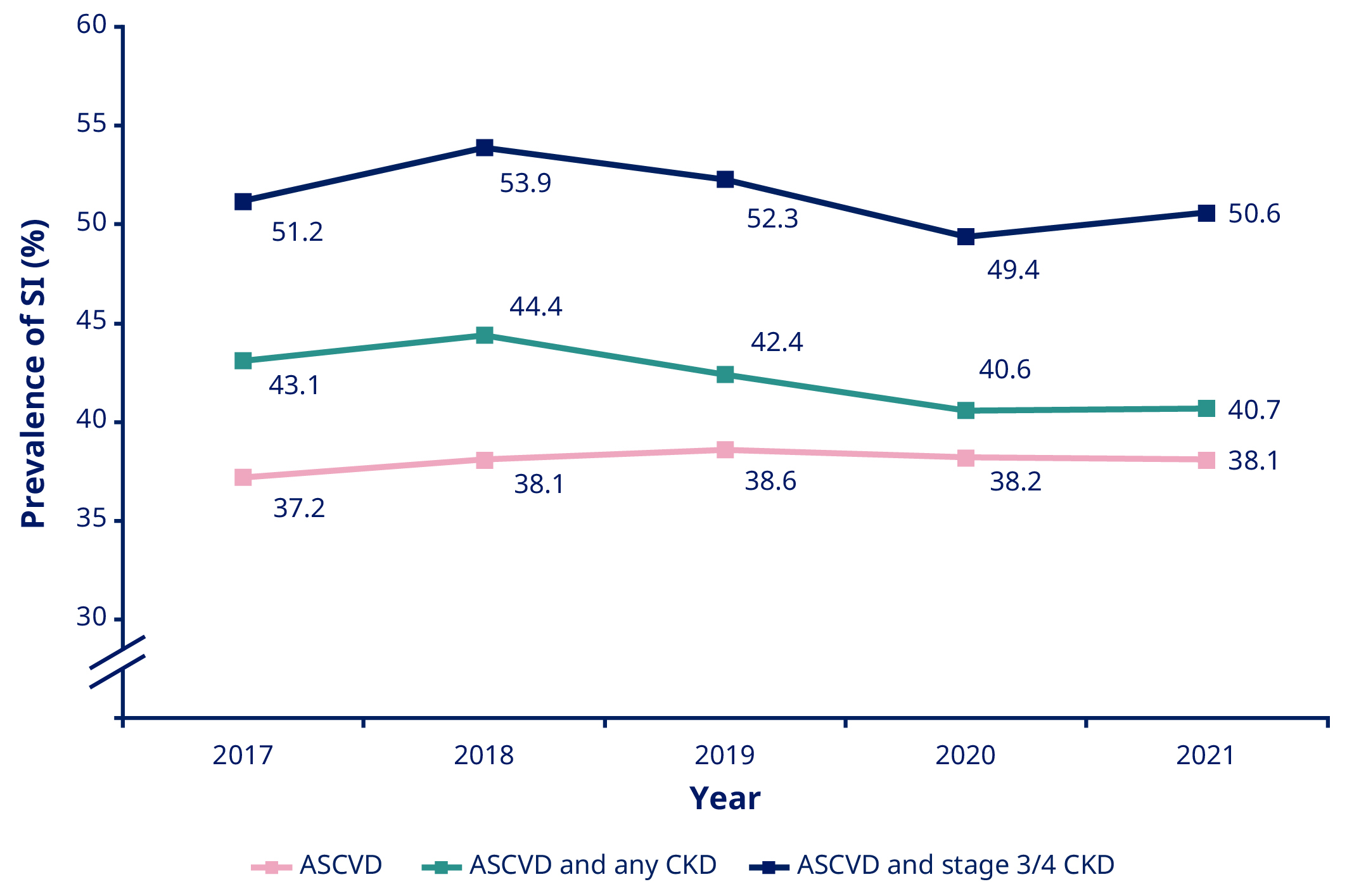
Results: Across the study period, prevalence of hsCRP testing among patients with ASCVD remained relatively stable (0.87–0.98%). Among patients who underwent hsCRP testing, SI was present in 38.0%, 42.3% and 51.5% of patients with ASCVD, ASCVD with CKD, and ASCVD with stage 3 or 4 CKD averaged across the study period, respectively (Figure 1). The prevalence of SI remained largely unchanged over the study period across the three groups (Figure 1). Among those with SI, a higher Charlson Comorbidity Index was noted in those with CKD: 0.75–0.78 in those with ASCVD, 0.99–1.21 in those with ASCVD and CKD, and 1.71–1.89 in those with ASCVD and stage 3 or 4 CKD. The most common medications utilized in those with SI were lipid-lowering therapy (70.5% [ASCVD], 72.7% [ASCVD with any CKD] and 75.4% [ASCVD with stage 3 or 4 CKD]) and antihypertensive therapy (72.8% [ASCVD], 79.3% [ASCVD with any CKD] and 86.7% [ASCVD with stage 3 or 4 CKD]) averaged across the study period.
Conclusion: Among patients with ASCVD undergoing hsCRP testing, SI was common, particularly among those with stage 3 or 4 CKD. This finding may help identify at-risk individuals who are likely to benefit from treatment of SI.
Aim: To define the prevalence and clinical characteristics of patients with SI and ASCVD with or without chronic kidney disease (CKD).
Methods: A retrospective cross-sectional analysis of US adults (aged ≥18 years) with any diagnoses of ASCVD evaluated with a hsCRP test using the Optum® de-identified electronic health record dataset between 2017 and 2021. SI was defined as an hsCRP level of 2–10 mg/L. The prevalence of SI was evaluated by calendar year, stratified to three groups by presence of ASCVD, ASCVD with any stage of CKD, and ASCVD with stage 3 or 4 CKD. Concomitant comorbidities and medications used were assessed and stratified by CKD severity.
Results: Across the study period, prevalence of hsCRP testing among patients with ASCVD remained relatively stable (0.87–0.98%). Among patients who underwent hsCRP testing, SI was present in 38.0%, 42.3% and 51.5% of patients with ASCVD, ASCVD with CKD, and ASCVD with stage 3 or 4 CKD averaged across the study period, respectively (Figure 1). The prevalence of SI remained largely unchanged over the study period across the three groups (Figure 1). Among those with SI, a higher Charlson Comorbidity Index was noted in those with CKD: 0.75–0.78 in those with ASCVD, 0.99–1.21 in those with ASCVD and CKD, and 1.71–1.89 in those with ASCVD and stage 3 or 4 CKD. The most common medications utilized in those with SI were lipid-lowering therapy (70.5% [ASCVD], 72.7% [ASCVD with any CKD] and 75.4% [ASCVD with stage 3 or 4 CKD]) and antihypertensive therapy (72.8% [ASCVD], 79.3% [ASCVD with any CKD] and 86.7% [ASCVD with stage 3 or 4 CKD]) averaged across the study period.
Conclusion: Among patients with ASCVD undergoing hsCRP testing, SI was common, particularly among those with stage 3 or 4 CKD. This finding may help identify at-risk individuals who are likely to benefit from treatment of SI.
More abstracts on this topic:
A 3-Year, Pre-Trial, Real-world Data Analysis of Patients Enrolled in VICTORION-INITIATE: Insights Using Tokenization
Rodriguez Fatima, Cosmatos Irene, Desai Nihar, Wright R, Ross Elsie, Ali Yousuf, Kumar Biswajit, Han Guangyang, Cai Beilei, Abbas Cheryl, Ryan Amy
Anticoagulation For Patients On Hemodialysis And Atrial FibrillationEbrahimi Ramin, Alvarez Carlos, Dennis Paul