Final ID: MDP1396
Clinical outcomes of cardiac synchronization with or without an implantable cardioverter defibrillator based on pooled data from 5 clinical trials: a patient-level meta-analysis
Aims: To compare the clinical outcomes of CRT-D vs CRT-P using data from 5 landmark CRT trials, both overall and stratified by etiology of cardiomyopathy (ischemic vs non-ischemic), sex (male vs female), age (≥ 70 y/o vs < 70 y/o), and QRS morphology (IVCD, LBBB, RBBB).
Methods: We performed a meta-analysis of patient level data from 5 prospective CRT trials (MIRACLE, REVERSE, RAFT, COMPANION and MADIT-CRT). Inclusion criteria were CRT-P vs CRT-D status (randomized comparison only in COMPANION), age ≥ 18 y/o and LVEF ≤ 35%. Exclusion criteria included secondary prevention ICD, QRS < 120ms, pacemaker upgrade, ventricular pacing indication, or missing data. Primary outcome was composite of time to heart failure hospitalization (HFH) or all-cause death. Secondary outcomes were time to HFH and death. Outcomes were analyzed using a frequentist Cox Proportional Hazards mixed effects model adjusted for 17 variables.
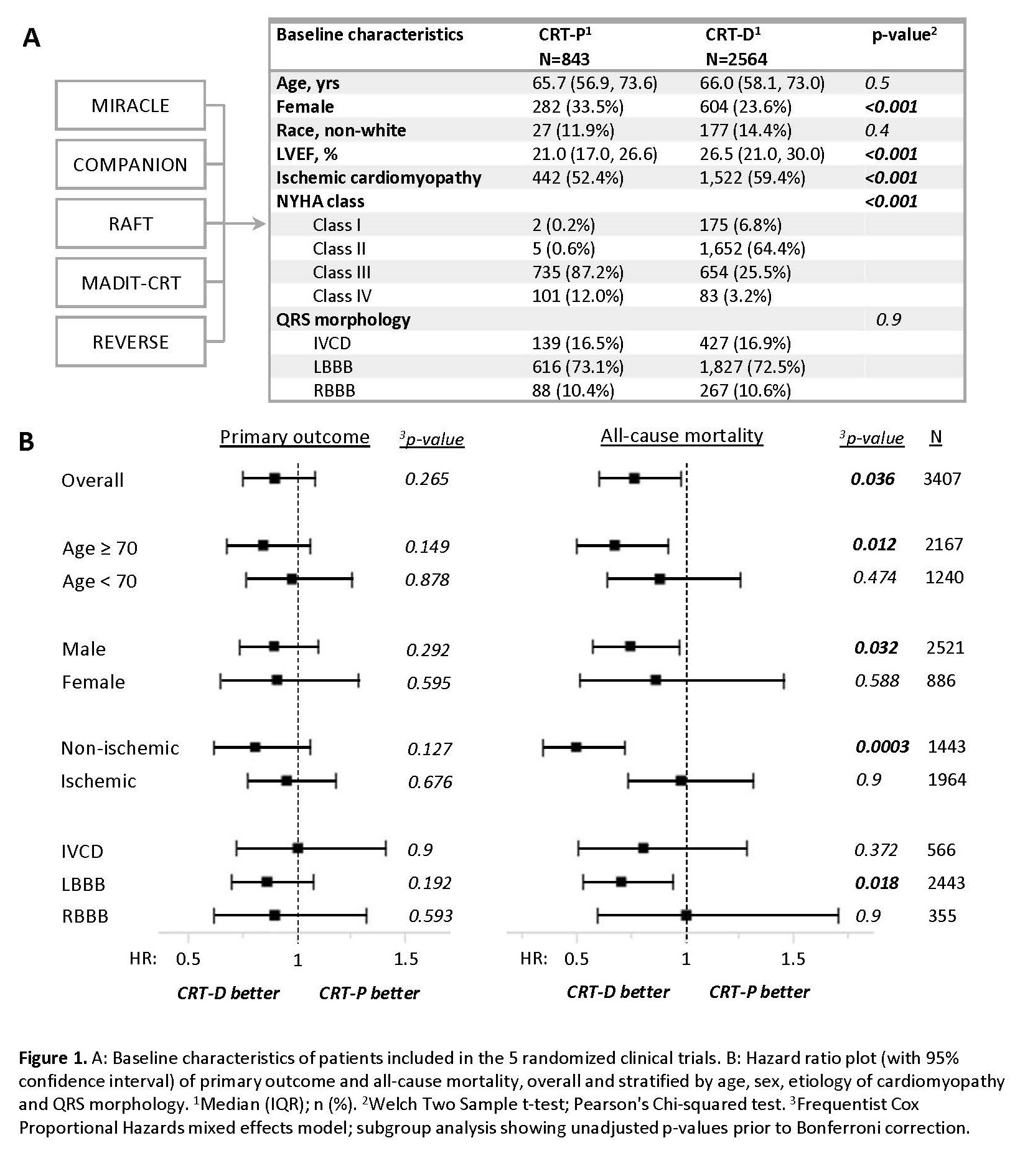
Results: A total of 3407 patients met inclusion criteria. Relative to patients with CRT-P (n=843), those with CRT-D (n=2564) were of similar age (66 y/o, p=0.5), less often female (24% vs 34%, p<0.001), and more often had ischemic cardiomyopathy (59.4% vs 52.4%, p<0.001), Fig 1A. Primary outcome was similar across groups (HR 0.902 [0.752, 1.081], p=0.26), but all-cause mortality was lower with CRT-D vs CRT-P (HR 0.77 [0.603, 0.983], p=0.036), Fig 1B. Interaction analyses suggested lower all-cause mortality with CRT-D vs CRT-P in patients with non-ischemic cardiomyopathy (HR 0.502 [0.346, 0.726], p=0.0003) and patients age ≥70 y/o (HR 0.679 [0.502, 0.919], p=0.012), with significance preserved after Bonferroni correction (Fig 1B).
Conclusion: In patients receiving CRT for HFrEF, those with CRT-D had lower all-cause mortality than patients with CRT-P, driven mainly by a lower mortality with CRT-D in older patients and those with non-ischemic cardiomyopathy. No significant interactions were noted between ICD & sex or ICD & QRS morphology.
- Shadrin, Ilya ( DUKE UNIVERSITY MEDICAL CENTER , Durham , North Carolina , United States )
- Kutyifa, Valentina ( UNIVERSITY OF ROCHESTER , Rochester , New York , United States )
- Linde, Cecilia ( Karolinska Institutet , Stockholm , Sweden )
- Young, James ( CLEVELAND CLINIC , Chagrin Falls , Ohio , United States )
- Tang, Anthony ( Western University , London , Ontario , Canada )
- Al-khatib, Sana ( DUKE UNIVERSITY MEDICAL CENTER , Durham , North Carolina , United States )
- Inoue, Lurdes ( University of Washington , Seattle , Washington , United States )
- Schmidler, Gillian ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Mackenzie, Michael ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Friedman, Daniel ( DUKE UNIVERSITY MEDICAL CENTER , Durham , North Carolina , United States )
- Abraham, William ( Ohio State University , Powell , Ohio , United States )
- Cleland, John ( University of Glasgow , Glasgow , United Kingdom )
- Curtis, Anne ( University at Buffalo , Buffalo , New York , United States )
- Gold, Michael ( MEDICAL UNIVERSITY SOUTH CAROLINA , Charleston , South Carolina , United States )
Meeting Info:
Session Info:
What Can Big Data Teach Us About Heart Failure
Monday, 11/18/2024 , 12:50PM - 02:15PM
Moderated Digital Poster Session
More abstracts on this topic:
Dabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to MyofibroblastsNatarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan
More abstracts from these authors:
Kutyifa Valentina, Tung Roderick, Ellenbogen Kenneth
When Should You Select Extravascular ICD vs Transvenous ICD?Birgersdotter-green Ulrika, Kutyifa Valentina, Friedman Paul