Final ID: Mo4060
CAR-T-associated Cardiovascular Events are Associated with Increased Resource Utilization
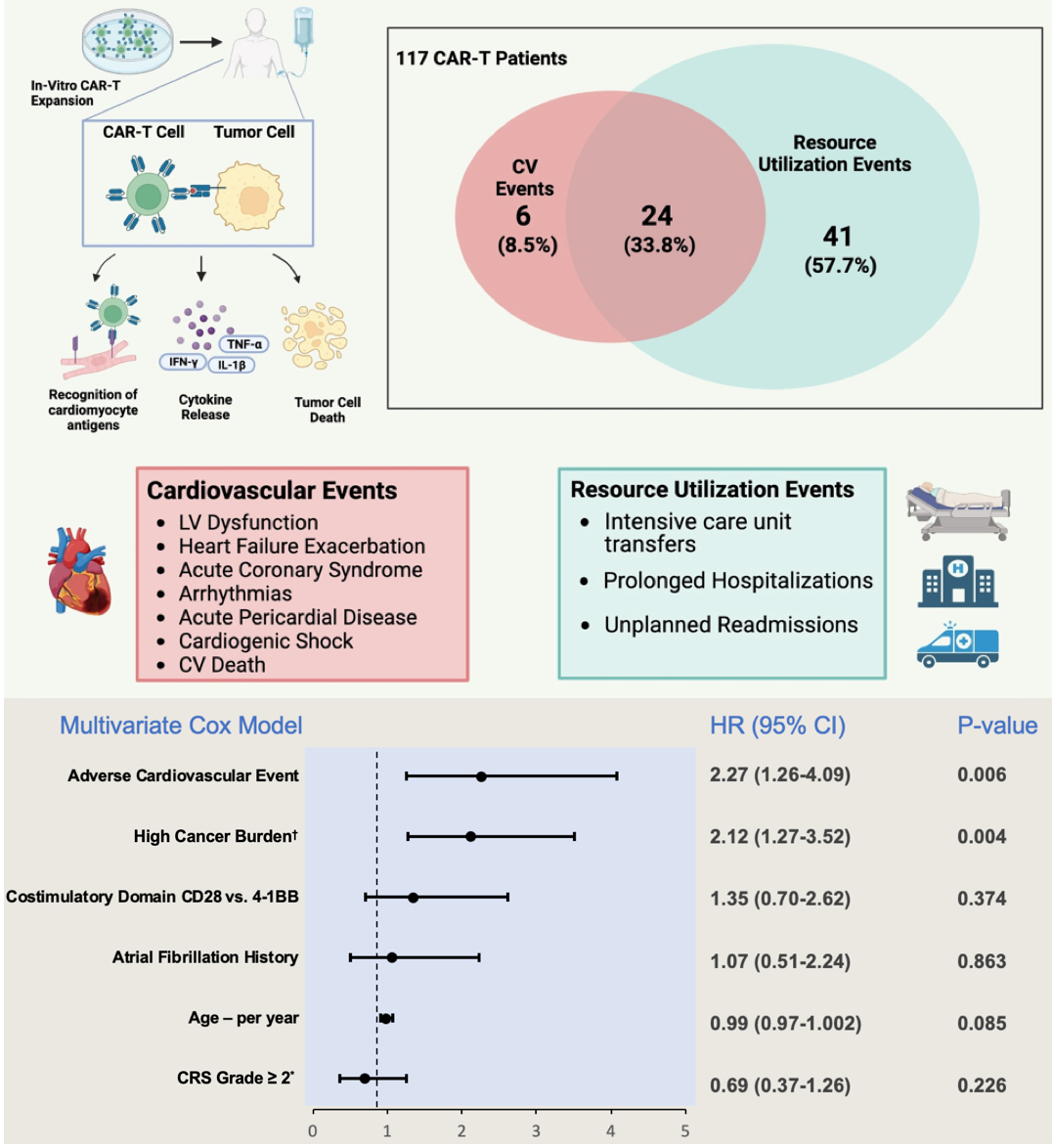
OBJECTIVE: To evaluate whether CAR-T-associated cardiovascular events are associated with RUE.
METHODS: Records of patients treated with an FDA-approved CAR-T product between 2018 and 2022 at a single institution were reviewed. Cardiovascular events post-CAR-T were defined as arrhythmia, acute coronary syndrome, stroke, cardiovascular death, new cardiomyopathy, heart failure, or pericarditis. RUE was a composite of intensive care unit (ICU) transfer, prolonged length of stay (LOS; >75th cohort percentile), and unplanned 6-month readmission.
RESULTS: Of 117 patients, 30 (26%) experienced cardiovascular events over a median 16 (IQR 7-34) months follow-up. RUE occurred in 65 patients (56%): 28 (24%) ICU transfers, 23 (20%) prolonged LOS, and 48 (41%) readmissions. Those with RUE experienced subsequent higher mortality (62% vs. 21%, p≤0.001). Among patients who experienced cardiovascular events, 24 (80%) experienced RUEs. In a Cox model, cardiovascular events (HR 2.3, 95% CI 1.3-4.1) and high cancer burden (HR 2.1, 95% CI 1.3-3.5) were independently associated with increased RUE rate, after adjusting for age, Cytokine Release Syndrome grade ≥2, atrial fibrillation history, and CAR-T costimulatory domain.
CONCLUSION: CAR-T recipients who experience cardiovascular events also experience increased resource utilization events. Further prospective studies are needed to assess whether post-CAR-T cardiovascular event management and/or pre-therapy assessment may reduce resource utilization burden.
- Song, Justin ( UCLA Medical Center , Los Angeles , California , United States )
- Eradat, Herbert ( UCLA Medical Center , Los Angeles , California , United States )
- Schiller, Gary ( UCLA Medical Center , Los Angeles , California , United States )
- Devos, Sven ( UCLA Medical Center , Los Angeles , California , United States )
- Timmerman, John ( UCLA Medical Center , Los Angeles , California , United States )
- Stein-merlob, Ashley ( UCLA Medical Center , Los Angeles , California , United States )
- Neilan, Tomas ( MASSACHUSETTS GENERAL HOSPITAL , Boston , Massachusetts , United States )
- Larson, Sarah ( UCLA Medical Center , Los Angeles , California , United States )
- Young, Patricia ( UCLA Medical Center , Los Angeles , California , United States )
- Mahmood, Syed ( Saint Francis Hospital & Heart Center , Roslyn , New York , United States )
- Yang, Eric ( UCLA Medical Center , Los Angeles , California , United States )
- Vuong, Jacqueline ( Cedars-Sinai Medical Center , Los Angeles , California , United States )
- Mead, Monica ( UCLA Medical Center , Los Angeles , California , United States )
- Gornbein, Jeffrey ( UCLA Medical Center , Los Angeles , California , United States )
- Rothberg, Michael ( UCLA Medical Center , Los Angeles , California , United States )
- Pan, Chelsea ( UCLA Medical Center , Los Angeles , California , United States )
- Dhaliwal, Jasmeet ( UCLA Medical Center , Los Angeles , California , United States )
- Boiarsky, Jonathan ( UCLA Medical Center , Los Angeles , California , United States )
- Gaut, Daria ( UCLA Medical Center , Los Angeles , California , United States )
Meeting Info:
Session Info:
Ace of MACE: Major Adverse Cardiovascular Events in Cardio-Oncology
Monday, 11/18/2024 , 01:30PM - 02:30PM
Abstract Poster Session
More abstracts on this topic:
Chiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin
Antigen Presenting Cell-Specific Keap1-Nrf2 Pathway Mediates Salt-Sensitive HypertensionKhan Mohd, Saleem Mohammad, Kirabo Annet
More abstracts from these authors:
Stein-merlob Ashley, Kondapalli Lavanya, Neilan Tomas, Asnani Aarti
A Rare Case of Testicular Diffuse Large B-Cell Lymphoma with Cardiac InvolvementJain Raina, Hu Xuchen, Boiarsky Jonathan, Hwang Sophia, Sehl Mary, Sedrak Mina, Pullarkat Sheeja, Yang Eric