Final ID: MDP401
Changes in Contrast Volume among Patients Undergoing Percutaneous Coronary Intervention: a Report from NCDR CathPCI registry
Abstract Body (Do not enter title and authors here): Background: Acute kidney injury (AKI) is the most common complication after percutaneous coronary intervention (PCI) and can be mitigated by using less iodinated contrast. The National Cardiovascular Data Registry (NCDR) supports quality improvement initiatives by providing quarterly, risk-adjusted, institutional AKI rates. However, it remains uncertain whether these efforts have reduced procedural contrast volume, particularly in high-risk patients.
Aims: Examine temporal trends in contrast volume during PCI across different pre-procedural AKI risk strata.
Methods: Within the NCDR CathPCI Registry, we identified PCIs performed between April 1, 2018, and December 31, 2022, and examined changes in mean contrast volume over time across different pre-procedural AKI risk strata defined by the validated NCDR AKI risk model. A multivariable hierarchical model, with the physician as a random effect, was constructed to assess the trend in contrast volume use over time.
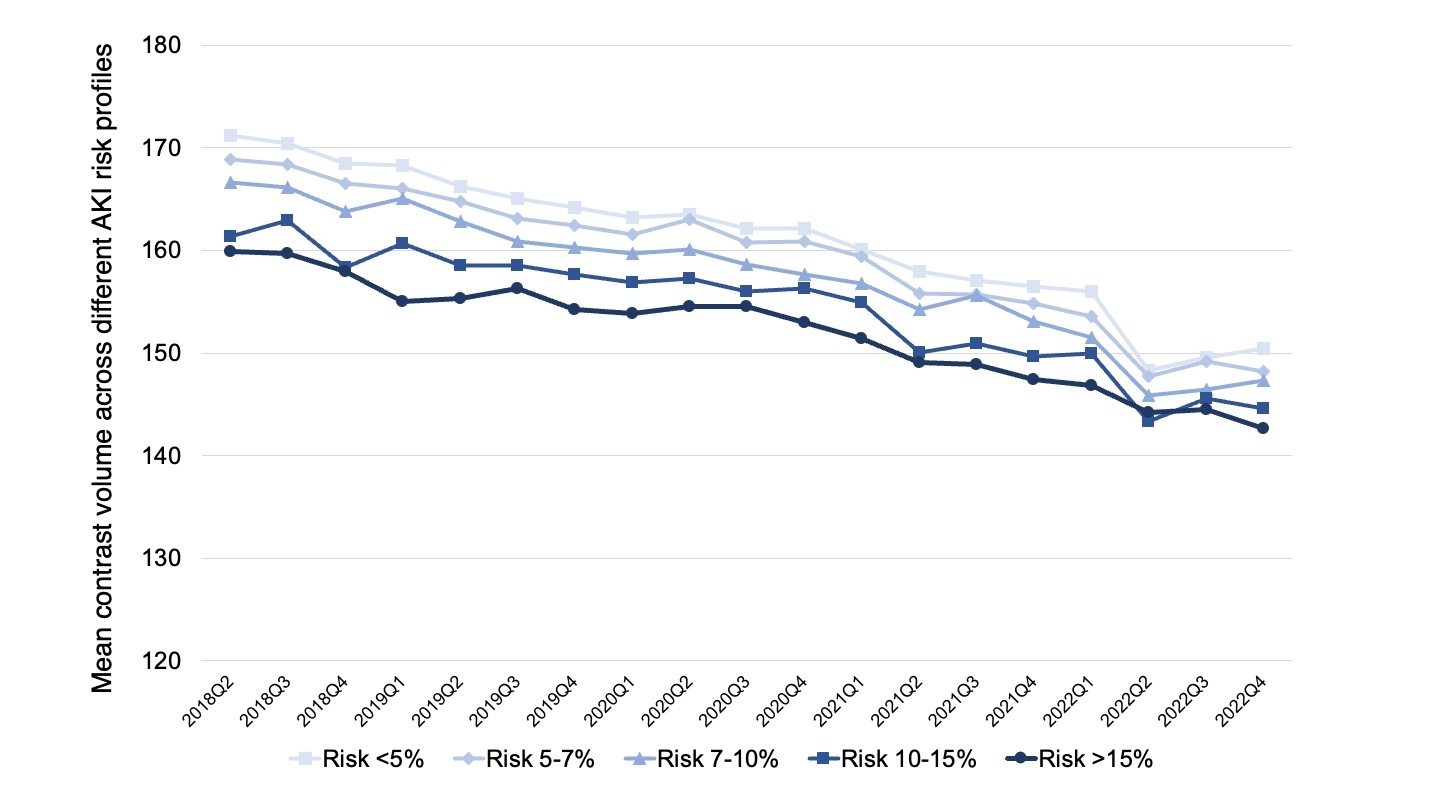
Results: Among 3,126,559 patients undergoing PCI (mean age = 66.8 ± 11.7 years, 69.4% men, and 39.9% elective procedures), contrast volume declined from a mean of 168.1±77.6 ml in Q2 2018 to 149.8±71.2 ml in Q4 2022 (p< 0.001 for trend) with the trend being consistent across AKI risk strata (Figure). After adjusting for patient and pre-procedural characteristics, the contrast volume declined by 18.9 ml (95%CI 19.1 to 18.6, P<0.001) between 2018 and 2022. Despite marked physician variability in mean contrast use, the lowest mean contrast doses were observed in patients at greatest AKI risk (>15%).
Conclusions: PCI contrast administration has declined over time, and the average exposure was lowest in patients at highest risk for AKI, suggesting improved efforts to reduce AKI over time.
Aims: Examine temporal trends in contrast volume during PCI across different pre-procedural AKI risk strata.
Methods: Within the NCDR CathPCI Registry, we identified PCIs performed between April 1, 2018, and December 31, 2022, and examined changes in mean contrast volume over time across different pre-procedural AKI risk strata defined by the validated NCDR AKI risk model. A multivariable hierarchical model, with the physician as a random effect, was constructed to assess the trend in contrast volume use over time.
Results: Among 3,126,559 patients undergoing PCI (mean age = 66.8 ± 11.7 years, 69.4% men, and 39.9% elective procedures), contrast volume declined from a mean of 168.1±77.6 ml in Q2 2018 to 149.8±71.2 ml in Q4 2022 (p< 0.001 for trend) with the trend being consistent across AKI risk strata (Figure). After adjusting for patient and pre-procedural characteristics, the contrast volume declined by 18.9 ml (95%CI 19.1 to 18.6, P<0.001) between 2018 and 2022. Despite marked physician variability in mean contrast use, the lowest mean contrast doses were observed in patients at greatest AKI risk (>15%).
Conclusions: PCI contrast administration has declined over time, and the average exposure was lowest in patients at highest risk for AKI, suggesting improved efforts to reduce AKI over time.
More abstracts on this topic:
Acute Stroke Treatment Metrics and Outcomes in Telestroke vs Non-Telestroke Care within the Paul Coverdell Michigan Stroke-Registry
Stamm Brian, Scott Phillip, Sheth Kevin, Reeves Mathew, Levine Deborah, Whitney Rachael, Royan Regina, Ibrahim Ghada, Nickles Adrienne, Ferber Rebecca, Hsu Wan-ling, Hayward Rodney, Mcdermott Mollie
An individual patient data meta-analysis of complete versus culprit-lesion only revascularization for acute myocardial infarction involving >8,800 individuals: The Complete Revascularization Trialists’ CollaborationMehta Shamir, Banning Amerjeet, Ramasundarahettige Chinthanie, Nguyen Helen, Wood David, Engstrom Thomas, Tiong Denise, Böhm Felix, James Stefan, Biscaglia Simone, Campo Gianluca, Smits Pieter, Giacoppo Daniele, Mccann Gerry