Final ID: MDP1249
HBI-3000: Pharmacological Conversion of Atrial Fibrillation With Unique Defense Against Excessive QT Interval Prolongation
Abstract Body (Do not enter title and authors here): Background: HBI-3000 (Sulcardine sulfate) is a new antiarrhythmic drug (AAD) that inhibits multiple cardiac ion channels (INa-peak, INa-late, ICa,L and IKr (hERG)) with uniquely similar potencies. A major AAD limitation is malignant proarrhythmia due to excessive hERG-mediated delay in repolarization, manifested by QT prolongation with proportional J to T peak [JTp] prolongation. While sulcardine inhibits hERG, it also possesses an intrinsic protective mechanism, inhibiting inward INa-late and ICa,L early repolarization currents. In Phase 1 volunteers in sinus rhythm (SR) therapeutic levels of sulcardine not only reduced but reversed the lengthening of the rate-corrected JTp (JTpc), reducing the extent of hERG-related QT prolongation. We investigated this protective JTpc effect in atrial fibrillation (AF) patients at the anticipated clinical dose, and examined the relationships between plasma concentration, QT prolongation, and the JTPc reduction.
Methods: Ten patients received 350 mg HBI-3000 IV over 30 min in a Phase 2 open-label study of patients with acute AF. Sulcardine plasma concentrations and ECG intervals (from 5-min summary ECGs) were obtained at baseline and several time points. Mean baseline-subtracted, heart rate-corrected QTcF (dQTcF) and JTp (dJTpc; Johannesen’s method) were calculated at each time point. AF patient data were compared to those of volunteers in SR.
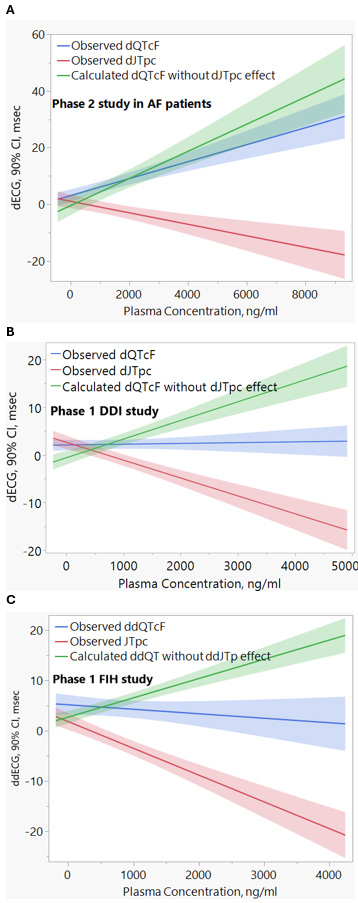
Results: In AF patients, dQTcF prolonged with a linear relationship to plasma concentration as expected (Fig 1A, blue). HBI-3000 IV produced a large linear reduction in JTpc contribution to the QT interval (Fig 1A, red). This is consistent with findings in SR (Fig 1B [350 mg dose] and 1C [360 mg dose]) and unique among AADs that inhibit IKr along with INa-late and/or ICa,L. Calculated dQTcF changes without the JTpc effect were greater than the observed dQTcF (Fig 1A, green) by 27%-76% at Cmax in the three studies.
Conclusions: HBI-3000 (Sulcardine) exhibits dQTcF prolongation protection in both AF and SR due to a unique pattern of channel fluxes that shortens early repolarization. This shorter dJTp interval acts as a mechanism for effectively limiting excessive QT prolongation and reducing proarrhythmic risk. [Registration: NCT04680026]
Methods: Ten patients received 350 mg HBI-3000 IV over 30 min in a Phase 2 open-label study of patients with acute AF. Sulcardine plasma concentrations and ECG intervals (from 5-min summary ECGs) were obtained at baseline and several time points. Mean baseline-subtracted, heart rate-corrected QTcF (dQTcF) and JTp (dJTpc; Johannesen’s method) were calculated at each time point. AF patient data were compared to those of volunteers in SR.
Results: In AF patients, dQTcF prolonged with a linear relationship to plasma concentration as expected (Fig 1A, blue). HBI-3000 IV produced a large linear reduction in JTpc contribution to the QT interval (Fig 1A, red). This is consistent with findings in SR (Fig 1B [350 mg dose] and 1C [360 mg dose]) and unique among AADs that inhibit IKr along with INa-late and/or ICa,L. Calculated dQTcF changes without the JTpc effect were greater than the observed dQTcF (Fig 1A, green) by 27%-76% at Cmax in the three studies.
Conclusions: HBI-3000 (Sulcardine) exhibits dQTcF prolongation protection in both AF and SR due to a unique pattern of channel fluxes that shortens early repolarization. This shorter dJTp interval acts as a mechanism for effectively limiting excessive QT prolongation and reducing proarrhythmic risk. [Registration: NCT04680026]
More abstracts on this topic:
A Novel JAK Activity Reporter for Live Cell Screening and Drug Discovery
Lopez-cecetaite Gabriel, Song Qianxiao, Severino Alex, Reyes Gaido Oscar, Luczak Elizabeth
Hacking the ubiquitin code to distinctively modulate ion channel functional expressionShanmugam Sri Karthika, Kanner Scott, Zou Xinle, Choudhury Papiya, Soni Rajesh, Kass Robert, Colecraft Henry