Final ID: 4136621
Efficacy of Empagliflozin in Heart Failure with Preserved Ejection Fraction is related to the presence of echocardiographic features of diastolic dysfunction in the EMPEROR-Preserved trial.
Abstract Body (Do not enter title and authors here): Introduction
Efficacy of SGLT2i empagliflozin in Heart Failure is seen across the LVEF spectrum and in multiple clinical subgroups, with diverse mechanisms of action. We hypothesized that objective echo evidence of diastolic dysfunction (DD) in HFpEF may predict the response to empagliflozin.
Methods
We defined DD as the presence of LAVI ≥34 mL/m2, E/e ≥13 or LVMi >115 (males) or >95 (females) and created three categories with 0, 1 or ≥2 DD criteria. We report the impact of baseline DD on response to empagliflozin therapy and adverse effects.
Analyses were performed using the intention-to-treat principle. For time-to-first-event analyses, differences in treatment effects across the three DD categories were assessed by a Cox proportional hazards model, with pre-specified covariates of age, gender, region, diabetes, LVEF, and eGFR. Total events were assessed using a joint frailty model, with CV death (CVD) as a competing risk.
Results
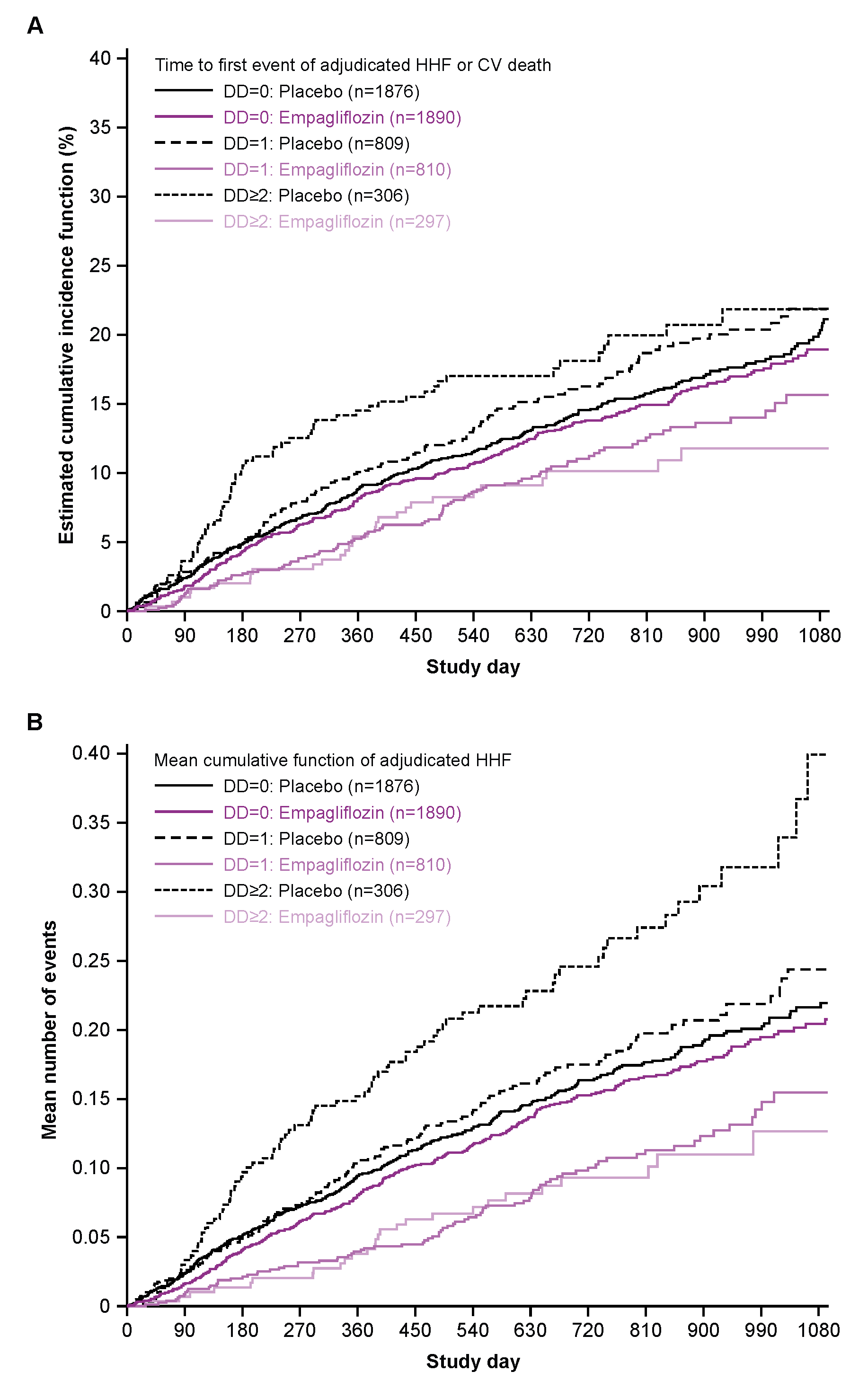
Of 5988 patients in EMPEROR-Preserved 3766 (63%) had no baseline DD, 1619 (27%) one and 603 (10%) ≥2 measures. The percentage of males decreased as DD category increased, 57.2% for none, 54.2% for one and 46.6% for ≥2, p <0.0001. Other demographic features significantly associated with DD categories were race, ethnicity and region and it was more common with age, with lower HR, DBP, body weight and eGFR. DD was more frequent with ischaemic aetiology and higher LVEF, but not with baseline NT−proBNP, DM or COPD. Time to HHF or CVD showed a progressive treatment effect across the three DD categories (interaction p-value 0.0014), with estimated HR’s of 0.91 (none present), 0.64 (1 present) and 0.49 (>=2). A significant treatment-by-subgroup interaction was also observed for the total number of HHF, with estimated HR’s of 0.95 (none present), 0.52 (1 present) and 0.29 (≥2).
In the responder analysis, ≥10 point improvement in KCCQ at week 52 showed a treatment induced change that was consistent across DD categories (interaction p-value 0.92). The safety profile was consistent across DD classes.
Conclusions
Baseline echocardiographic DD predicted an improved clinical response to Empagliflozin in a graded fashion, suggesting the mode of action may target specific changes in diastolic performance.
Efficacy of SGLT2i empagliflozin in Heart Failure is seen across the LVEF spectrum and in multiple clinical subgroups, with diverse mechanisms of action. We hypothesized that objective echo evidence of diastolic dysfunction (DD) in HFpEF may predict the response to empagliflozin.
Methods
We defined DD as the presence of LAVI ≥34 mL/m2, E/e ≥13 or LVMi >115 (males) or >95 (females) and created three categories with 0, 1 or ≥2 DD criteria. We report the impact of baseline DD on response to empagliflozin therapy and adverse effects.
Analyses were performed using the intention-to-treat principle. For time-to-first-event analyses, differences in treatment effects across the three DD categories were assessed by a Cox proportional hazards model, with pre-specified covariates of age, gender, region, diabetes, LVEF, and eGFR. Total events were assessed using a joint frailty model, with CV death (CVD) as a competing risk.
Results
Of 5988 patients in EMPEROR-Preserved 3766 (63%) had no baseline DD, 1619 (27%) one and 603 (10%) ≥2 measures. The percentage of males decreased as DD category increased, 57.2% for none, 54.2% for one and 46.6% for ≥2, p <0.0001. Other demographic features significantly associated with DD categories were race, ethnicity and region and it was more common with age, with lower HR, DBP, body weight and eGFR. DD was more frequent with ischaemic aetiology and higher LVEF, but not with baseline NT−proBNP, DM or COPD. Time to HHF or CVD showed a progressive treatment effect across the three DD categories (interaction p-value 0.0014), with estimated HR’s of 0.91 (none present), 0.64 (1 present) and 0.49 (>=2). A significant treatment-by-subgroup interaction was also observed for the total number of HHF, with estimated HR’s of 0.95 (none present), 0.52 (1 present) and 0.29 (≥2).
In the responder analysis, ≥10 point improvement in KCCQ at week 52 showed a treatment induced change that was consistent across DD categories (interaction p-value 0.92). The safety profile was consistent across DD classes.
Conclusions
Baseline echocardiographic DD predicted an improved clinical response to Empagliflozin in a graded fashion, suggesting the mode of action may target specific changes in diastolic performance.
More abstracts on this topic:
β-alanine Supplementation Improves Diastolic Function in Heart Failure with preserved Ejection Fraction
Doelling Benjamin, Chaudhari Mamata, Makhloufi Jamila, Hoetker David, Brittian Kenneth, Nong Yibing, Baba Shahid
A Rare Cause of Recurrent Heart Failure Exacerbations After Transcatheter Aortic Valve Replacement: Ventricular Septal Defect and Significant Paravalvular LeakMedina Jesse, Vincent Louis, Rodriguez Ferreira Esteban, Spence-miller Shanice, Fernandez Joel, Colombo Rosario, Calfa Marian