Final ID: MDP166
Prematurity Predicts Neurodevelopmental Outcomes in Congenital Heart Disease
Abstract Body (Do not enter title and authors here): Background: Neurodevelopmental (ND) impairment is common in both prematurity and congenital heart disease (CHD). Preterm infants with CHD may have a “double hit,” with worse ND impairments than seen in prematurity or CHD alone. We analyzed whether gestational age (GA) at delivery predicted ND outcomes in children with CHD.
Methods: We performed a single-institution retrospective study of children who underwent infant heart surgery from 2007-2020 and returned for ND evaluation after discharge. Exclusion criteria were 1) trisomy 21 and 2) PDA ligation alone. The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) assessed ND between ages 6-40 months. Patients were stratified into 4 GA groups: very/moderately preterm (≤33 weeks), late-preterm (34-36 weeks), early-term (37-38 weeks) or term/post-term (≥39 weeks). Hierarchical linear regression evaluated potential predictors of Bayley-III composite scores including GA category, demographic, medical, and surgical variables.
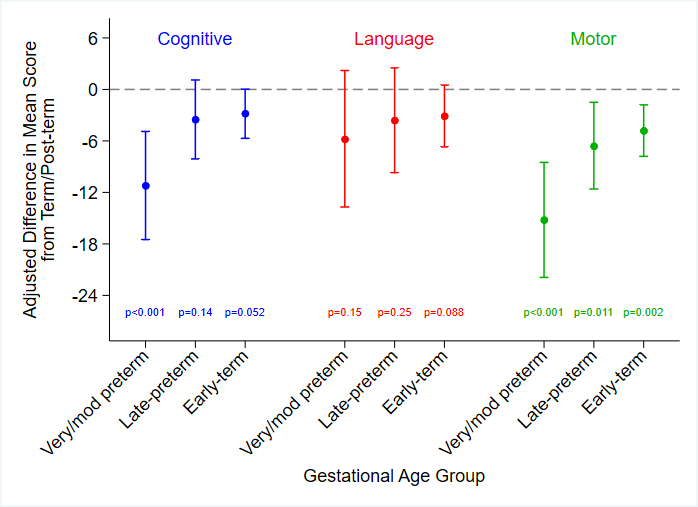
Results: Among 1350 infants, 513 (38%) had a Bayley-III at median age 28 months [IQR 19, 34]. Of these, 448 (87%) were full-term and 65 (13%) preterm. In univariate analyses, children born preterm vs. full term had lower Bayley-III cognitive, language, and motor scores, explaining from 2.4% (language) to 6.2% (motor) of the variance in Bayley composite scores. In the final models, GA remained a strong independent predictor of motor and cognitive scores. Children with GA ≤33 weeks had the worst outcomes, with age-adjusted cognitive and motor composite scores 11 and 15 points lower, respectively, than those of term infants (Figure). Other significant risk factors included lower primary caregiver education, Black race, longer hospital length of stay, and genetic diagnosis. These models ultimately explained 17-26% of the variance in Bayley scores, with the highest explained variance in the motor domain.
Conclusions: Adjusting for other patient and medical factors, preterm infants with CHD have worse cognitive and motor outcomes; risk is highest in those born at ≤33 weeks. Further work is needed to identify potentially modifiable factors to tailor prenatal, postnatal and perioperative care of infants with CHD born prematurely.
Methods: We performed a single-institution retrospective study of children who underwent infant heart surgery from 2007-2020 and returned for ND evaluation after discharge. Exclusion criteria were 1) trisomy 21 and 2) PDA ligation alone. The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) assessed ND between ages 6-40 months. Patients were stratified into 4 GA groups: very/moderately preterm (≤33 weeks), late-preterm (34-36 weeks), early-term (37-38 weeks) or term/post-term (≥39 weeks). Hierarchical linear regression evaluated potential predictors of Bayley-III composite scores including GA category, demographic, medical, and surgical variables.
Results: Among 1350 infants, 513 (38%) had a Bayley-III at median age 28 months [IQR 19, 34]. Of these, 448 (87%) were full-term and 65 (13%) preterm. In univariate analyses, children born preterm vs. full term had lower Bayley-III cognitive, language, and motor scores, explaining from 2.4% (language) to 6.2% (motor) of the variance in Bayley composite scores. In the final models, GA remained a strong independent predictor of motor and cognitive scores. Children with GA ≤33 weeks had the worst outcomes, with age-adjusted cognitive and motor composite scores 11 and 15 points lower, respectively, than those of term infants (Figure). Other significant risk factors included lower primary caregiver education, Black race, longer hospital length of stay, and genetic diagnosis. These models ultimately explained 17-26% of the variance in Bayley scores, with the highest explained variance in the motor domain.
Conclusions: Adjusting for other patient and medical factors, preterm infants with CHD have worse cognitive and motor outcomes; risk is highest in those born at ≤33 weeks. Further work is needed to identify potentially modifiable factors to tailor prenatal, postnatal and perioperative care of infants with CHD born prematurely.
More abstracts on this topic:
β1 integrins regulate cellular behavior and cardiomyocyte organization during ventricular wall formation
Miao Lianjie, Schwartz Robert, R Burns Alan, Kumar Ashok, Dipersio C. Michael, Wu Mingfu, Lu Yangyang, Nusrat Anika, Zhao Luqi, Castillo Micah, Xiao Yongqi, Guo Hongyan, Liu Yu, Gunaratne Preethi
A Tale of Two Surgeries: Single Stage Versus Two Staged Approach to Creating an Ovine Model of Fontan PalliationKievert Jennifer, Morrison Adrienne, Nelson Kirsten, Heuer Eric, Shinoka Toshiharu, Breuer Christopher, Carrillo Sergio Alejandro, Kelly John, Spiess Joshua, Deshetler Cameron, Yuhara Satoshi, Watanabe Tatsuya, Jimenez Michael, Naguib Aymen, Mckee Christopher, Yates Andrew