Final ID: 4134695
The Effect and Mechanism of Histone Lactylation In Pathological Cardiac Hypertrophy Induced by Pressure Overload
Abstract Body (Do not enter title and authors here): Introduction: Cardiac hypertrophy is characterized by significant metabolic changes, notably an increase in glycolysis. Histone lactylation, a post-translational modification influenced by the glycolytic state of cells, plays a crucial role in regulating gene transcription. However, the relationship between histone lactylation and cardiac hypertrophy remains unclear.
Research Questions: This study aims to elucidate the effects and underlying mechanisms of histone lactylation in the progression of cardiac hypertrophy induced by pressure overload.
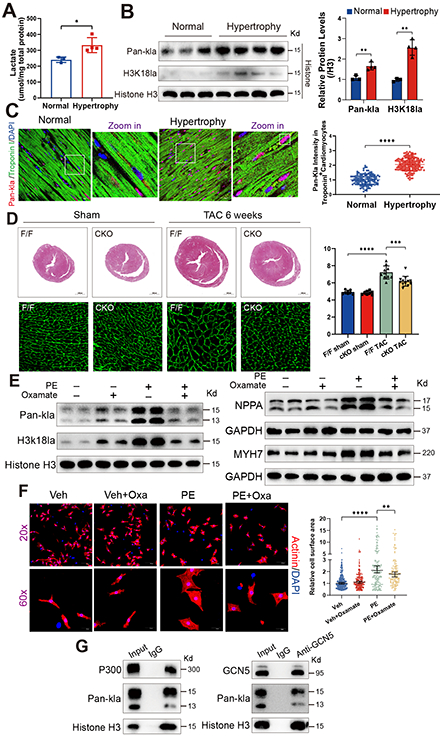
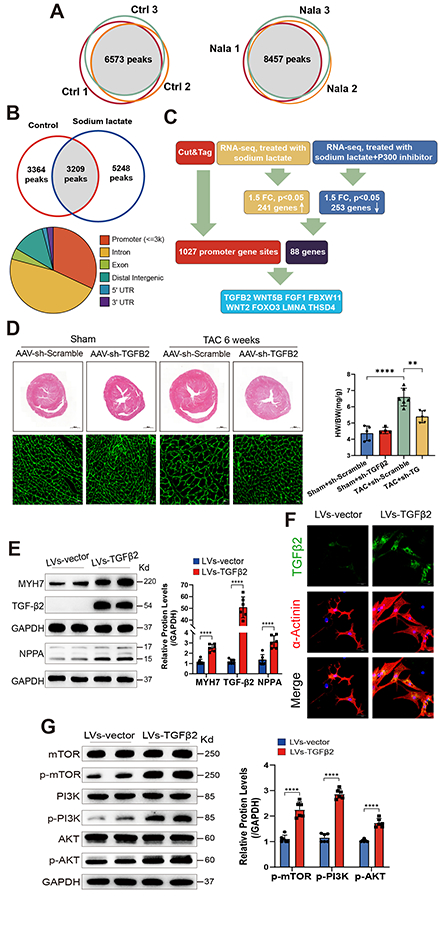
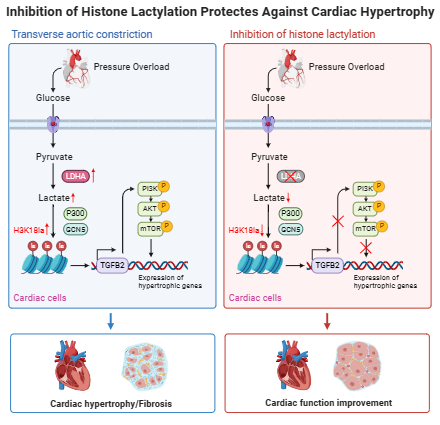
Methods and Results: We observed an increase in histone lactylation levels in hypertrophic hearts from both humans and mice. To investigate further, male mice underwent transverse aortic constriction (TAC) to induce cardiac hypertrophy, followed by either an intraperitoneal injection of Oxamate (an LDHA inhibitor) to reduce histone lactylation or sodium lactate to increase it. Reducing histone lactylation protected the hearts from TAC-induced hypertrophy and fibrosis, preserving cardiac function, while increased histone lactylation exacerbated cardiac hypertrophy and fibrosis, impairing cardiac function. Cardiomyocyte-specific deletion of LDHA also led to a reduction in cardiac hypertrophy. In vitro experiments showed that inhibiting histone lactylation reduced the expression of hypertrophic markers and the hypertrophic growth of cardiomyocytes stimulated by phenylephrine. Using co-immunoprecipitation, we confirmed that P300 and GCN5 are the transferases mediating histone lactylation in cardiomyocytes. Mechanistic studies using the CUT&Tag technique revealed lactate-dependent histone modification was enriched at the promoter of TGFB2, activating its transcription. Inhibiting cardiac-specific TGFB2 expression through adeno-associated viral delivery of shRNA reduced TAC-induced cardiac hypertrophy. Furthermore, TGFB2 overexpression induced a hypertrophic phenotype in cardiomyocytes and activated the PI3K/AKT/mTOR pathways. This hypertrophic phenotype, induced by TGFB2 overexpression, was suppressed by inhibitors of PI3K or AKT.
Conclusion: Our findings reveal a critical role for histone lactylation in the development of pathological cardiac hypertrophy by regulating TGFB2 expression and the PI3K/AKT/mTOR pathway.
Research Questions: This study aims to elucidate the effects and underlying mechanisms of histone lactylation in the progression of cardiac hypertrophy induced by pressure overload.
Methods and Results: We observed an increase in histone lactylation levels in hypertrophic hearts from both humans and mice. To investigate further, male mice underwent transverse aortic constriction (TAC) to induce cardiac hypertrophy, followed by either an intraperitoneal injection of Oxamate (an LDHA inhibitor) to reduce histone lactylation or sodium lactate to increase it. Reducing histone lactylation protected the hearts from TAC-induced hypertrophy and fibrosis, preserving cardiac function, while increased histone lactylation exacerbated cardiac hypertrophy and fibrosis, impairing cardiac function. Cardiomyocyte-specific deletion of LDHA also led to a reduction in cardiac hypertrophy. In vitro experiments showed that inhibiting histone lactylation reduced the expression of hypertrophic markers and the hypertrophic growth of cardiomyocytes stimulated by phenylephrine. Using co-immunoprecipitation, we confirmed that P300 and GCN5 are the transferases mediating histone lactylation in cardiomyocytes. Mechanistic studies using the CUT&Tag technique revealed lactate-dependent histone modification was enriched at the promoter of TGFB2, activating its transcription. Inhibiting cardiac-specific TGFB2 expression through adeno-associated viral delivery of shRNA reduced TAC-induced cardiac hypertrophy. Furthermore, TGFB2 overexpression induced a hypertrophic phenotype in cardiomyocytes and activated the PI3K/AKT/mTOR pathways. This hypertrophic phenotype, induced by TGFB2 overexpression, was suppressed by inhibitors of PI3K or AKT.
Conclusion: Our findings reveal a critical role for histone lactylation in the development of pathological cardiac hypertrophy by regulating TGFB2 expression and the PI3K/AKT/mTOR pathway.
More abstracts on this topic:
Apelin Signaling Protects Against Experimental Pulmonary Hypertension-Induced Right Ventricular Remodeling Through Regulation of the Renin Angiotensin Aldosterone System
Bharti Manisha, Yakubov Bakhtiyor, Zagorski John, Albrecht Marjorie, Fisher Amanda, Cook Todd, Frump Andrea
Acetylation of Electron Transfer Flavoprotein Alpha Is a Possible Regulatory Mechanism of Fatty Acid Oxidation in Diabetic HeartsTatekoshi Yuki, Yano Masaki, Hosoda Ryusuke, Saga Yukika, Kuno Atsushi