Final ID: Sa1079
Hybrid Cardiomyocyte Targeting Peptide Down-regulates NF-κB Pathway in Human Cardiomyocytes
Abstract Body (Do not enter title and authors here): Introduction: Role of NF-κB driven inflammation leading to heart failure with preserved ejection fraction has received little attention. Our previous in vivo work with cardiac targeting peptide (CTP) in guinea pigs identified suppression of NF- κB pathway by CTP. In the current work we sought to further study the effects of a hybrid version of CTP (hCTP: amino acids at positions 3 and 11 substituted with D-amino acids) on TNF-α mediated NF-κB activation, as well as expression of its downstream inflammatory markers IL-1β and IL-8.
Methods: Human cardiomyocytes (CMCs) were transfected using Lipofectamine 3000 with a reporter luciferase plasmid carrying an NF-κB promoter site upstream of the luciferase gene. Cells were treated with various concentrations of hCTP for 24 hours, followed by a TNF-α challenge (20ng/mL), addition of luciferin as substrate, and measurement of luminescence. Cell viability in response to above manipulations was assessed by FACS using live-dead stain. CMCs were treated as above, and cell culture supernatants analyzed for IL-8 levels using human ELISA kit. In another set of experiments, CMCs were transfected with NF-κB reporter plasmid, and after treatment for 24 hours with various concentrations of hCTP, incubated with 10nM angiotensin 2 (Ang2) and 100nM phenylephrine (PE) for 24hrs to assess levels of IL-1β in the cell culture media using an ELISA assay.
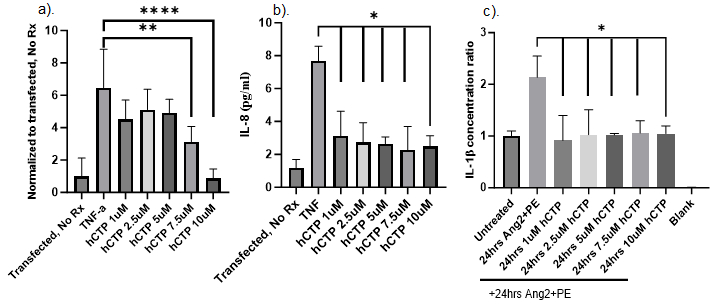
Results: hCTP inhibited TNF-α mediated NF-κB activation with a dose-response curve, with maximum inhibition with hCTP seen at 10µM concentrations (p<0.001). Treatment with hCTP inhibited the TNF-α induced IL-8 secretion with significant decreases seen even at the 1µM dose. Similarly, secretion of IL-1β production by CMCs in response to Ang2/PE stimulation was suppressed by hCTP even at the lowest tested dose (1µM; p<0.5).
Conclusions: Our data suggests that hCTP inhibited NF-κB pathway transcriptional activity in response to TNF-α stimulation in a dose-dependent fashion in human CMCs. Furthermore, treatment with hCTP inhibited TNF-α induced IL-8 and IL-1β secretion even at doses as low as 1µM. The ability of hCTP to efficiently targeting this inflammatory pathway suggests that it has therapeutic potential in the treatment of heart failure. Further in vivo studies are ongoing to test this hypothesis.
Methods: Human cardiomyocytes (CMCs) were transfected using Lipofectamine 3000 with a reporter luciferase plasmid carrying an NF-κB promoter site upstream of the luciferase gene. Cells were treated with various concentrations of hCTP for 24 hours, followed by a TNF-α challenge (20ng/mL), addition of luciferin as substrate, and measurement of luminescence. Cell viability in response to above manipulations was assessed by FACS using live-dead stain. CMCs were treated as above, and cell culture supernatants analyzed for IL-8 levels using human ELISA kit. In another set of experiments, CMCs were transfected with NF-κB reporter plasmid, and after treatment for 24 hours with various concentrations of hCTP, incubated with 10nM angiotensin 2 (Ang2) and 100nM phenylephrine (PE) for 24hrs to assess levels of IL-1β in the cell culture media using an ELISA assay.
Results: hCTP inhibited TNF-α mediated NF-κB activation with a dose-response curve, with maximum inhibition with hCTP seen at 10µM concentrations (p<0.001). Treatment with hCTP inhibited the TNF-α induced IL-8 secretion with significant decreases seen even at the 1µM dose. Similarly, secretion of IL-1β production by CMCs in response to Ang2/PE stimulation was suppressed by hCTP even at the lowest tested dose (1µM; p<0.5).
Conclusions: Our data suggests that hCTP inhibited NF-κB pathway transcriptional activity in response to TNF-α stimulation in a dose-dependent fashion in human CMCs. Furthermore, treatment with hCTP inhibited TNF-α induced IL-8 and IL-1β secretion even at doses as low as 1µM. The ability of hCTP to efficiently targeting this inflammatory pathway suggests that it has therapeutic potential in the treatment of heart failure. Further in vivo studies are ongoing to test this hypothesis.
More abstracts on this topic:
A Novel H2 Relaxin B-Chain-Only Peptide Variant B7-33 Improves The Pathophysiology Of Placental Ischemia In The Reduced Uterine Perfusion Pressure Rat Model Of Preeclampsia
Pantho Ahmed F, Hossain Mohammed, Uddin Mohammad, Amaral Lorena, Campbell Nathan, Afroze Syeda, Vora Niraj, Kuehl Thomas, Lindheim Steven, Lamarca Babbette, Bathgate Ross
A major uremic toxin indoxyl sulfate impairs macrophage efferocytosis and accelerates atherogenesis: a potential mechanism for cardiovascular risk in chronic kidney diseaseJha Prabhash, Kasai Taku, Vromman Amelie, Holden Rachel, Libby Peter, Tabas Ira, Singh Sasha, Aikawa Elena, Aikawa Masanori, Lupieri Adrien, Sonawane Abhijeet, Le Thanh-dat, Becker-greene Dakota, Chelvanambi Sarvesh, Turner Mandy, Nakamura Yuto, Passos Livia