Final ID: MDP339
Socioeconomic status, race, and ethnicity in management of pediatric SVT
Abstract Body (Do not enter title and authors here): Introduction: Electrophysiology study and ablation (EPS) is often the preferred approach to management of supraventricular tachycardia (SVT) and/or ventricular pre-excitation (WPW) in older children/adolescents. There are inequities in arrhythmia management in adults, and in other areas of pediatric cardiology, but little is known about disparities in management of SVT in children.
Research question: Do racial/ethnic and socioeconomic inequities affect referral for EPS in patients with SVT and/or WPW?
Methods: This retrospective observational study utilized the Pediatric Health Information System of 49 children’s hospitals’ administrative data from 2016-2022. We included patients with SVT or WPW seen in the emergency department, admitted for observation, or inpatient. We excluded patients with congenital heart disease. The outcome was having a procedure code for EPS. The childhood opportunity index (COI, scale 1-100) based on zip code was used to measure socioeconomic status (SES).
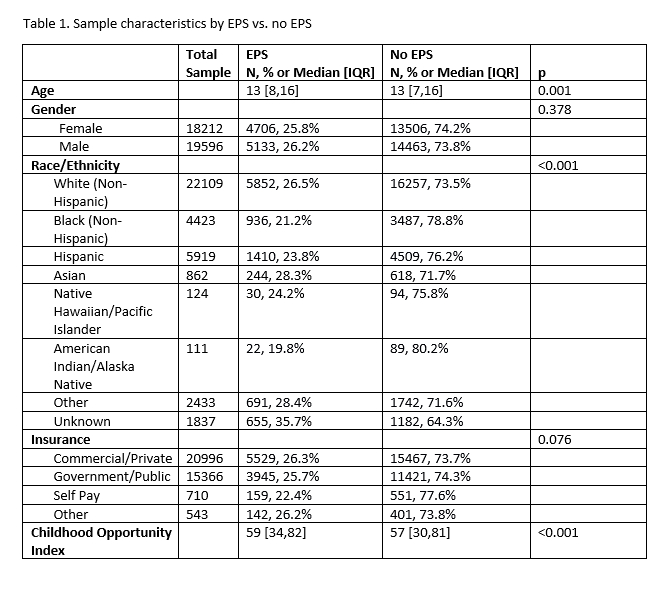
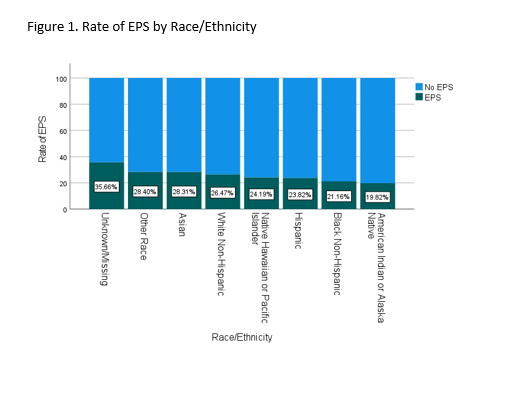
Results: Among 37,818 patients with primary diagnosis of SVT (27620, 73%) or WPW (10198, 27%), 9840 (26%) underwent EPS (Table 1). COI was higher in patients who underwent EPS than those who did not (median 59 [IQR 34,82] vs 57 [IQR 30,81]). In addition, time from initial visit with diagnosis of SVT/WPW to EPS was longer in patients with lower COI (beta= -0.026, p=0.007). Black (21.2%), Hispanic (23.8%), and American Indian (19.8%) patients were less likely to undergo EPS than White (26.5%) and Asian (28.31%) patients and those with other (28.4%) or unknown/missing (35.7%) race (p<0.001, Figure 1). In both subgroups of patients with SVT and WPW, EPS differed by race and COI, and higher COI predicted shorter time to EPS.
Conclusions: In this hospital database of pediatric patients, we found disparities in use of EPS for the management of SVT and/or WPW. Patients of minoritized race/ethnicity and lower SES were less likely to undergo EPS.
Research question: Do racial/ethnic and socioeconomic inequities affect referral for EPS in patients with SVT and/or WPW?
Methods: This retrospective observational study utilized the Pediatric Health Information System of 49 children’s hospitals’ administrative data from 2016-2022. We included patients with SVT or WPW seen in the emergency department, admitted for observation, or inpatient. We excluded patients with congenital heart disease. The outcome was having a procedure code for EPS. The childhood opportunity index (COI, scale 1-100) based on zip code was used to measure socioeconomic status (SES).
Results: Among 37,818 patients with primary diagnosis of SVT (27620, 73%) or WPW (10198, 27%), 9840 (26%) underwent EPS (Table 1). COI was higher in patients who underwent EPS than those who did not (median 59 [IQR 34,82] vs 57 [IQR 30,81]). In addition, time from initial visit with diagnosis of SVT/WPW to EPS was longer in patients with lower COI (beta= -0.026, p=0.007). Black (21.2%), Hispanic (23.8%), and American Indian (19.8%) patients were less likely to undergo EPS than White (26.5%) and Asian (28.31%) patients and those with other (28.4%) or unknown/missing (35.7%) race (p<0.001, Figure 1). In both subgroups of patients with SVT and WPW, EPS differed by race and COI, and higher COI predicted shorter time to EPS.
Conclusions: In this hospital database of pediatric patients, we found disparities in use of EPS for the management of SVT and/or WPW. Patients of minoritized race/ethnicity and lower SES were less likely to undergo EPS.
More abstracts on this topic:
A Multi-Center Clinic Site Comparison of Patient-level factors Affecting Oral Anticoagulation Prescription for Atrial Fibrillation
Iqbal Fatima, Hoang Kenneth, Chiadika Simbo
Adaptive Cardiac Arrest Training Curriculum for Capacity Building in Northern Ghana: Addressing Contextual Challenges for SustainabilityAhadzi Dzifa, Boateng Laud, Hernandez Odalys Rivera, Akanbong Prosper, Leung Claudia, Al-hassan Rahma, Baba Yabasin Iddrisu, Yakubu Abdul-subulr, Cournooh Annette, Ikeda Scott, Alomatu Samuel, Sakeah Patience