Final ID: Mo3126
The Safety Profile Of Inclisiran In Patients With Dyslipidemia And Intermediate To High Atherosclerotic Cardiovascular Disease Risk: A Systematic Review And Meta-Analysis
Abstract Body (Do not enter title and authors here): Introduction: Inclisiran is a novel drug that employs ribonucleic acid (RNA) interference to lower levels of the proprotein convertase subtilisin/kexin type 9 (PCSK9) protein, thereby improving the clearance of low-density lipoprotein (LDL) cholesterol from the bloodstream. It is given through subcutaneous injections at specified intervals and significantly reduces LDL cholesterol levels in patients with dyslipidemia and with intermediate to high risk of atherosclerotic cardiovascular disease (ASCVD).
Goal: We aim to evaluate the safety of the use of inclisiran in patients with dyslipidemia and intermediate to high ASCVD risk.
Methods: Three electronic databases of MEDLINE, Web of Science, and Embase were searched from inception to May 5th 2024 to identify relevant randomized controlled trials (RCTs) comparing safety profiles of inclisiran versus the control group . The primary outcome was all-cause mortality. Secondary outcomes were major adverse cardiovascular events (MACE), injection-site reaction and all treatment-emergent adverse events (TEAE). The effect estimates of outcomes were assessed using risk ratio (RR) with a 95% confidence interval (CI). Random-effects meta-analysis was conducted using the restricted maximum likelihood method given the inter-study variance was inevitable. The subgroup analysis was performed based on different dosing regimens.
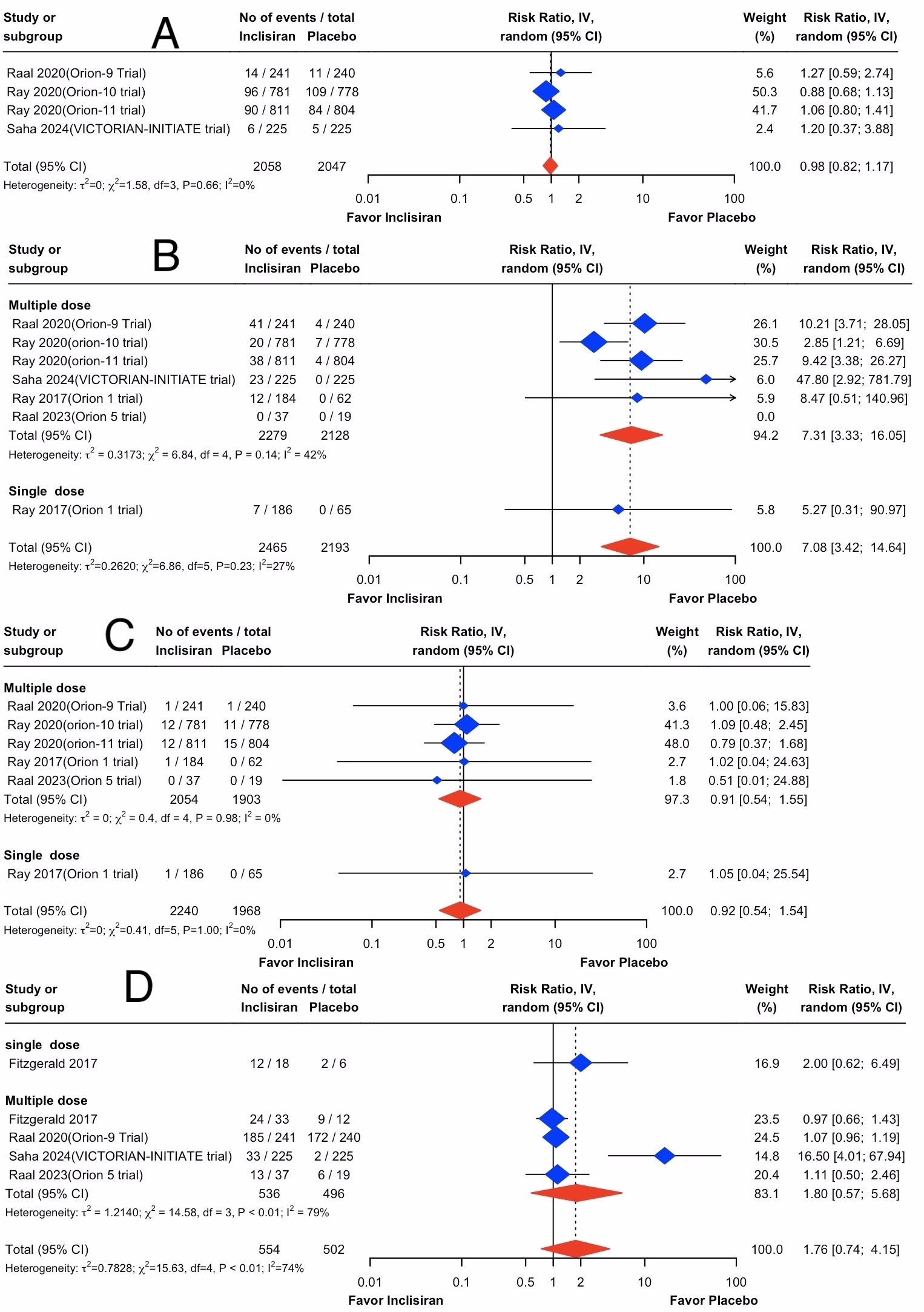
Results: We included 7 RCTs enrolling 4790 patients (63.8 ± 9.7 years, 66.8 % males) who received inclisiran. Compared to the control group, the use of inclisiran did not yield a significant effect on all-cause mortality (RR, 0.92; 95% CI, 0.54 to 1.54; I2 = 0%), MACE (RR, 0.98; 95% CI, 0.82 to 1.17; I2 = 0%), and TEAE (RR, 1.76; 95% CI, 0.74 to 4.15; I2 = 74%). However, inclisiran use significantly increased injection-site reaction (RR, 7.08; 95% CI, 3.42 to 14.64; I2 = 27%). Results are illustrated in the accompanying figure.
Conclusions: Inclisiran use significantly increased injection-site reaction, with no increase in mortality and TEAE. Further trials are required to comprehensively assess the safety of inclisiran in the management of dyslipidemia in patients with ASCVD risk.
Goal: We aim to evaluate the safety of the use of inclisiran in patients with dyslipidemia and intermediate to high ASCVD risk.
Methods: Three electronic databases of MEDLINE, Web of Science, and Embase were searched from inception to May 5th 2024 to identify relevant randomized controlled trials (RCTs) comparing safety profiles of inclisiran versus the control group . The primary outcome was all-cause mortality. Secondary outcomes were major adverse cardiovascular events (MACE), injection-site reaction and all treatment-emergent adverse events (TEAE). The effect estimates of outcomes were assessed using risk ratio (RR) with a 95% confidence interval (CI). Random-effects meta-analysis was conducted using the restricted maximum likelihood method given the inter-study variance was inevitable. The subgroup analysis was performed based on different dosing regimens.
Results: We included 7 RCTs enrolling 4790 patients (63.8 ± 9.7 years, 66.8 % males) who received inclisiran. Compared to the control group, the use of inclisiran did not yield a significant effect on all-cause mortality (RR, 0.92; 95% CI, 0.54 to 1.54; I2 = 0%), MACE (RR, 0.98; 95% CI, 0.82 to 1.17; I2 = 0%), and TEAE (RR, 1.76; 95% CI, 0.74 to 4.15; I2 = 74%). However, inclisiran use significantly increased injection-site reaction (RR, 7.08; 95% CI, 3.42 to 14.64; I2 = 27%). Results are illustrated in the accompanying figure.
Conclusions: Inclisiran use significantly increased injection-site reaction, with no increase in mortality and TEAE. Further trials are required to comprehensively assess the safety of inclisiran in the management of dyslipidemia in patients with ASCVD risk.
More abstracts on this topic:
A Model-Sharing Approach for Quality Improvement of Diabetes and Cardiovascular Disease
Elligers Kyle, Pollner Meghan, Overton Katherine, Congdon Michelle, Greenway Stacey, Lambro Patricia, Sadiku Steven, Schechter Rona, Whelan John, Pressley Bianca, Sednew Renee, Duckett Sara
A Novel RNA Interference Agent RN0191 Lowering Proprotein Convertase Subtilisin/Kexin Type 9, Low-density Lipoprotein Cholesterol and Other Lipid Biomarkers in Healthy Volunteers with Elevated LDL Cholesterol: A Randomized, Single-blind, Placebo-controlled, Phase 1 TrialWang Fangfang, Li Haiyan, Zeng Jie, Shi Yibin, Li Hongmei