Final ID: Sa4033
Restrictive or Liberal Blood Transfusion in Patients with Myocardial Infarction and Renal Insufficiency
Objectives: To evaluate outcomes of those with CKD randomized to RTT vs. LTT in the Myocardial Ischemia and Transfusion (MINT) trial (NCT02981407).
Methods: Among 3,495 MINT participants with non-missing creatinine (99.7%), we compared the baseline characteristics and outcomes at 30 days post-randomization of those individuals without CKD (N = 1279), CKD with eGFR 30-60 mL/min/1.73 m2 (N = 999), CKD with eGFR < 30 mL/min/1.73 m2 (N = 802), and CKD requiring dialysis (N = 415), both overall and by randomized transfusion strategy. Interaction terms for eGFR category by treatment assignment on each outcome were assessed.
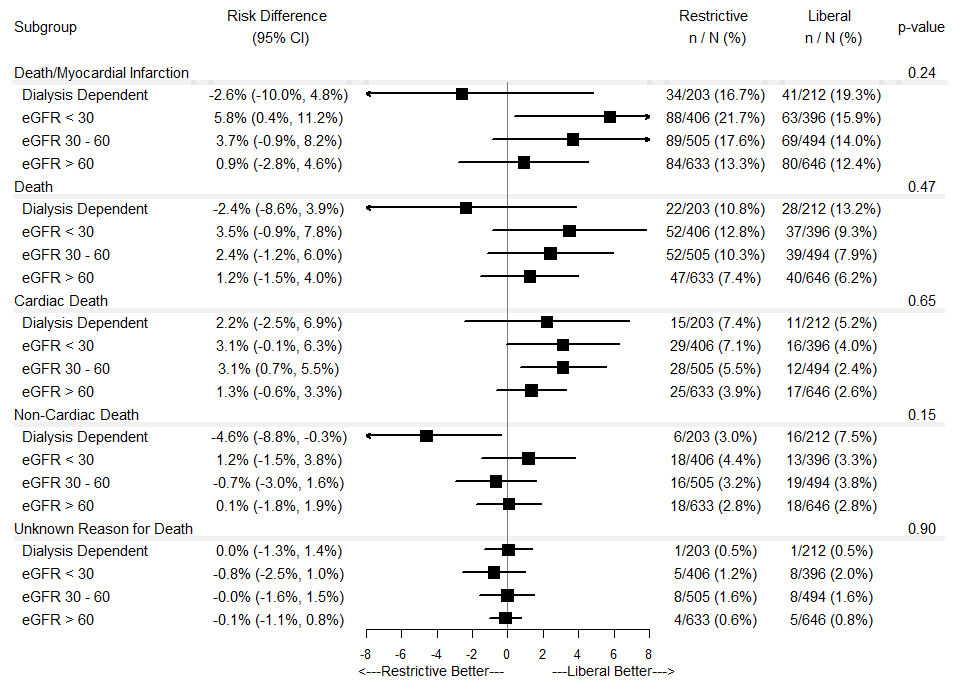
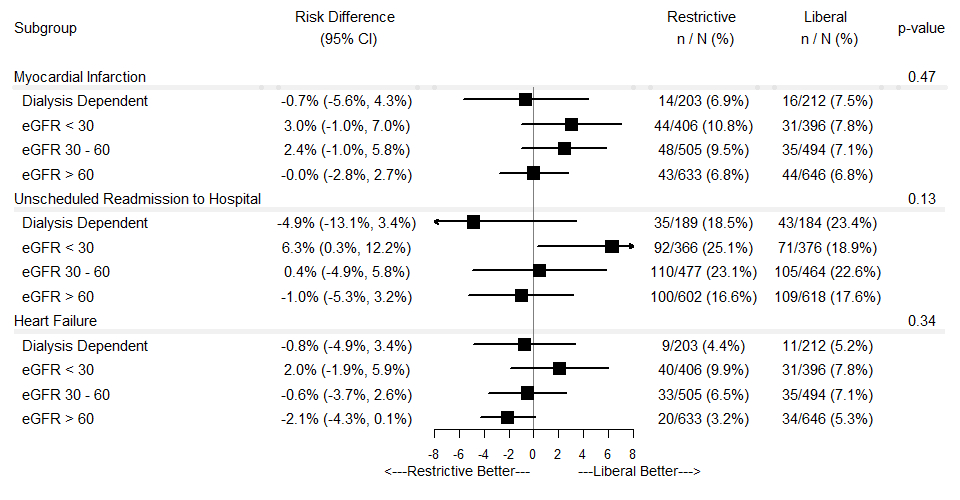
Results: Individuals with CKD compared to those without CKD more frequently presented with NSTEMI (all p < 0.001) and had a greater risk of all-cause death, recurrent MI, rehospitalization, and heart failure (all p < 0.05). Compared to a liberal transfusion strategy, a restrictive strategy among non-dialysis dependent individuals with an eGFR < 30 mL/min/1.73 m2 was associated with an increased risk of death/recurrent MI (Figure 1) and unplanned rehospitalization (Figure 2). Among individuals with an eGFR 30-60 mL/min/1.73 m2, a restrictive strategy was associated with an increased risk of cardiac death (Figure 1). No eGFR category by treatment assignment interaction terms were significant.
Conclusions: In this prespecified analysis, individuals with CKD were at greater risk of death, recurrent MI, heart failure, and unplanned rehospitalization at 30 days post-randomization than those without CKD. In individuals with CKD, a restrictive transfusion strategy was associated with increased risk of adverse outcomes.
- Strom, Jordan ( Beth Israel Deaconess Medical Center , Milton , Massachusetts , United States )
- Alsweiler, Caroline ( Green Lane Coordinating Centre , Auckland , New Zealand )
- Gupta, Rajesh ( University of Toledo Medical Center , Toledo , Ohio , United States )
- Ritt, Luiz Eduardo ( Bahiana School of Medicine , Salvador , Brazil )
- Menegus, Mark ( Montefiore Medical Center , Bronx , New York , United States )
- Alexander, John ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Lopes, Renato ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Chaitman, Bernard ( St. Louis University , Saint Louis , Missouri , United States )
- Carson, Jeffrey ( Rutgers Robert Wood Johnson Med , New Brunswick , New Jersey , United States )
- Bertolet, Marnie ( University of Pittsburgh , Allison Park , Pennsylvania , United States )
- Herbert, Brandon ( University of Pittsburgh , Allison Park , Pennsylvania , United States )
- Malik, Shahbaz ( University of Nebraska Medical Center , Omaha , Nebraska , United States )
- Lemesle, Gilles ( Institut Coeur Poumon, CHU de Lille , LILLE , France )
- Madan, Mina ( Sunnybrook Health Sciences Centre , Toronto , Ontario , Canada )
- Steg, Philippe ( Hopital Bichat , Paris , France )
- Traverse, Jay ( Minneapolis Heart Institute , Minneapolis , Minnesota , United States )
- White, Harvey ( Auckland City Hospital , Auckland , New Zealand )
Meeting Info:
Session Info:
Potpourri. A Variety of Issues Affecting the ACS Patient
Saturday, 11/16/2024 , 02:00PM - 03:00PM
Abstract Poster Session
More abstracts on this topic:
Vempati Roopeessh, Damarlapally Nanush, Vasudevan Srivatsa Surya, Banda Prathibha, Mourad Denise, Polamarasetty Harshavardhan, Mathur Gaurav, Khan Afrasayab, Desai Rupak
Ability of Composite Magnetic Resonance Brain Imaging Scores to Predict Functional Outcomes in Survivors of Cardiac ArrestNguyen Thuhien, Town James, Wahlster Sarah, Johnson Nicholas, Poilvert Nicolas, Lin Victor, Ukatu Hope, Matin Nassim, Davis Arielle, Taylor Breana, Thomas Penelope, Sharma Monisha
More abstracts from these authors:
Zeller Marianne, Coste Pierre, Mesnier Jules, Lemesle Gilles, Simon Tabassome, Danchin Nicolas, Bouleti Claire, Cottin Yves, Steg Philippe, Rousseau Alexandra, Lebal Soufiane, Frederic Frederic, Cayla Guillaume, Angoulvant Denis
Discussant: ADAPT AF-DESLemesle Gilles