Final ID: Sa2074
Influences of Age on Improvement of Health-Related Quality of Life in Patients with Atrial Fibrillation following Initial Treatment
Abstract Body (Do not enter title and authors here): Background: The prevalence of Atrial Fibrillation (AF), the most cardiac common arrhythmia, increases with age. Various treatment strategies are available to improve health-related quality of life (HRQoL) in patients with AF; however, the impact of age on HRQoL treatment benefits is unknown.
Aims: To investigate the association of age with HRQoL benefit across different treatment strategies.
Methods: Using data from a multicenter registry for outpatients with early recognized AF, HRQoL at enrollment and 1 year later was measured with the Atrial Fibrillation Effect on Quality-of-life quesTionnaire (AFEQT). The association between age and the 1-year changes in AFEQT-overall summary (OS) and subdomain scores with either rate or rhythm control strategies were assessed using generalized additive models (GAM) to address non-linear relationships. To support clinical interpretation, multivariable linear regression models were performed with categories of age ranges.
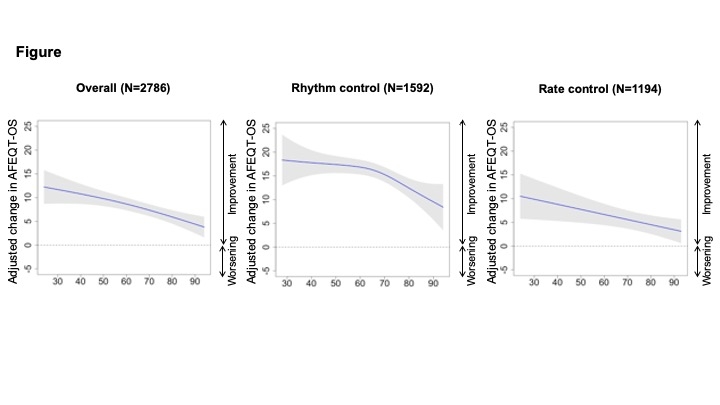
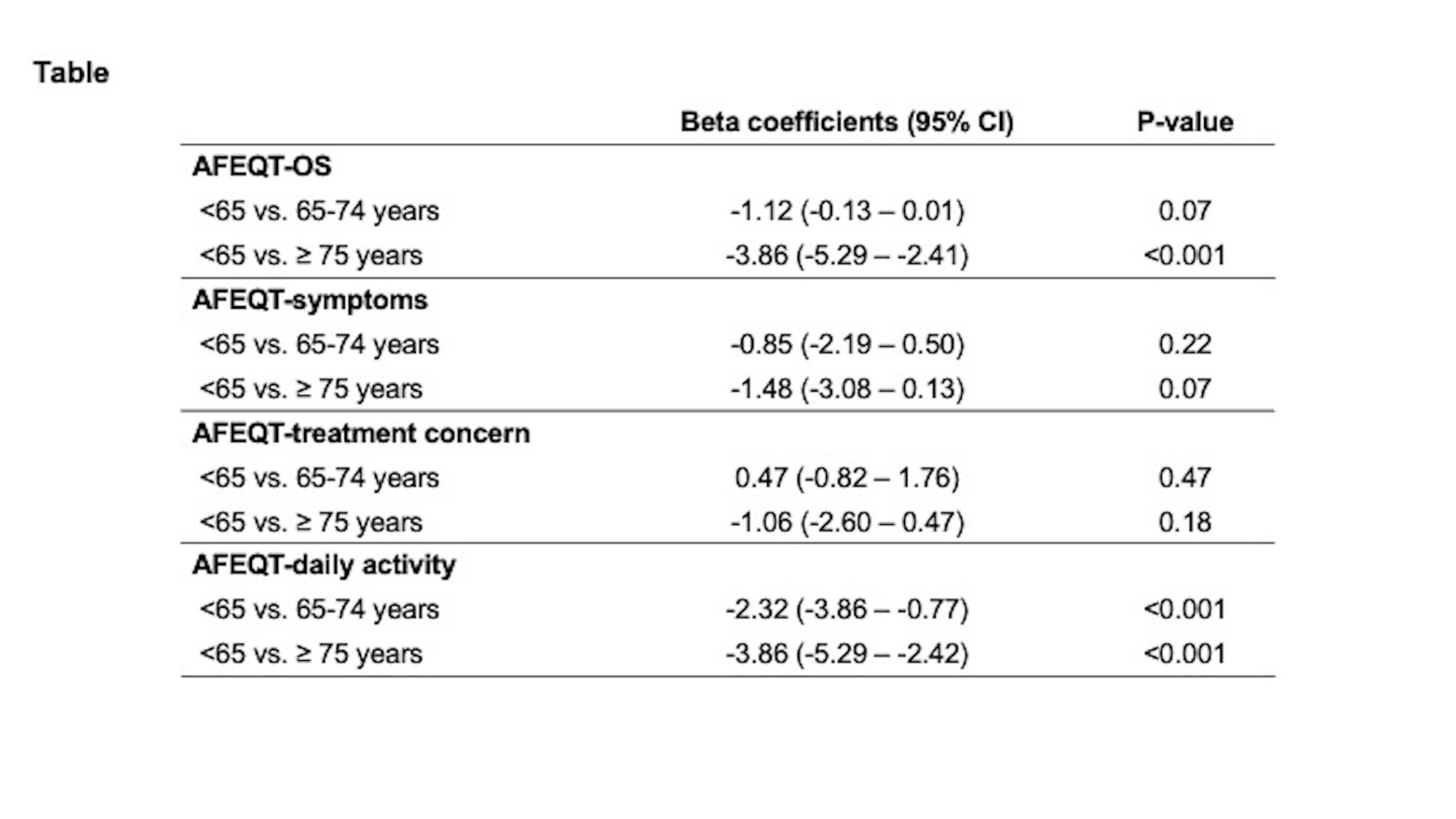
Results: Among 2786 patients (31.7% women, 54.7% rhythm control); 946 were <65 of age, 1019 were ≥65–75, and 821 were ≥75 years old. Rhythm (vs. rate) control was pursued in 76.6%, 58.0%, and 33.6% and mean AFEQT-OS changes were 11.1 (SD 16.6), 8.0 (17.0), and 4.7 (18.2) in those aged <65, 65–74, and ≥75, respectively. The GAM showed improvements in AFEQT-OS attenuated with advancing age with both treatment strategies (Figure). Linear regression analyses showed lesser improvements in AFEQT-OS as higher age categories (≥65–75 vs. <65, β -1.12 [95% CI, -2.33 – 0.09]; ≥75 vs. <65, β -3.86 [-5.29 – -2.42]), which were mainly driven by lesser improvement in AFEQT-daily activities scores, without differences in AFEQT scores for symptom or treatment concerns (Table).
Conclusions: In AF patients, HRQoL benefits after treatment were attenuated by advancing age, primarily driven by less improvement in functional limitations, underscoring the need for further research to identify optimal treatment strategies for older adults.
Aims: To investigate the association of age with HRQoL benefit across different treatment strategies.
Methods: Using data from a multicenter registry for outpatients with early recognized AF, HRQoL at enrollment and 1 year later was measured with the Atrial Fibrillation Effect on Quality-of-life quesTionnaire (AFEQT). The association between age and the 1-year changes in AFEQT-overall summary (OS) and subdomain scores with either rate or rhythm control strategies were assessed using generalized additive models (GAM) to address non-linear relationships. To support clinical interpretation, multivariable linear regression models were performed with categories of age ranges.
Results: Among 2786 patients (31.7% women, 54.7% rhythm control); 946 were <65 of age, 1019 were ≥65–75, and 821 were ≥75 years old. Rhythm (vs. rate) control was pursued in 76.6%, 58.0%, and 33.6% and mean AFEQT-OS changes were 11.1 (SD 16.6), 8.0 (17.0), and 4.7 (18.2) in those aged <65, 65–74, and ≥75, respectively. The GAM showed improvements in AFEQT-OS attenuated with advancing age with both treatment strategies (Figure). Linear regression analyses showed lesser improvements in AFEQT-OS as higher age categories (≥65–75 vs. <65, β -1.12 [95% CI, -2.33 – 0.09]; ≥75 vs. <65, β -3.86 [-5.29 – -2.42]), which were mainly driven by lesser improvement in AFEQT-daily activities scores, without differences in AFEQT scores for symptom or treatment concerns (Table).
Conclusions: In AF patients, HRQoL benefits after treatment were attenuated by advancing age, primarily driven by less improvement in functional limitations, underscoring the need for further research to identify optimal treatment strategies for older adults.
More abstracts on this topic:
Association between resilience and health status among adults after myocardial infarction
Chen Bryan, Liu Olivia, Bartelloni Alexis, Xia Yuhe, Reynolds Harmony, Spruill Tanya, Arabadjian Milla
Application of Digital Health Interventions in Quality of Life and Psychological Status of Stroke Patients: Systematic Review and Meta-analysisChen Lu, Shang Zhiying, He Manlan