Final ID: MDP1112
Empagliflozin Improves Mitochondrial Biogenesis in Pulmonary Arterial Hypertension
Abstract Body (Do not enter title and authors here): Background: Pulmonary arterial hypertension (PAH) is a devastating disease characterized by endothelial proliferation and progressive pulmonary vascular (PV) remodeling. Empagliflozin (EMPA), a sodium glucose cotransporter 2 (SGLT2) inhibitor, benefits heart failure patients, potentially mediated by improved mitochondrial function. This study aimed to explore the impact of EMPA in PAH.
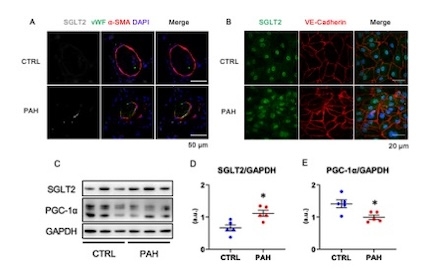
Methods and Results: Immunohistochemistry of pulmonary arteries revealed the expression of SGLT2 in the intima of PAH patients. Microvascular endothelial cells (MVECs) were isolated from lungs of control subjects (CTRL) and PAH patients and found intense expression of SGLT2 in PAH MVECs. Western blot showed significantly higher expression of SGLT2, while PGC-1α, a transcriptional coactivator known as a master regulator of mitochondrial biogenesis, was lower in PAH MVECs compared to CTRL (Image 1). PAH MVECs were treated with EMPA (1 μM) and observed a significant increase of PGC-1α and BMPR2-pSmad1/5/9. Similarly, knockdown of SGLT2 using siRNA resulted in enhanced expression of PGC-1α. Moreover, Seahorse analysis revealed increased oxygen consumption rate and reduced extracellular acidification rate by EMPA, suggesting that EMPA improved mitochondrial respiration. In addition, EMPA attenuated reactive oxygen species and proliferation of PAH MVECs, as revealed by MitoTracker analysis and MTT assay respectively.
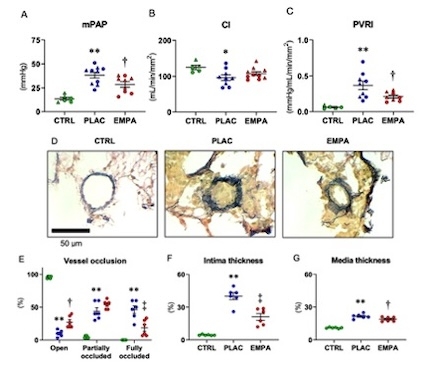
For the in vivo study, Sprague-Dawley rats (120-180 gram) were injected with SU5416 (Week-0) and exposed to hypoxia for 3 weeks followed by normoxia. After randomization at Week-4, rats were treated with EMPA (300 mg/kg in chow, n=12) or placebo (PLAC, n=12). Catheterization and histological analysis were performed at Week-8. EMPA decreased PV resistance index (0.21±0.02 vs. 0.37±0.05 mmHg/mL/min/mm2, p=0.01), ameliorated intima thickening (21.0±7.5 vs. 40.0±7.8%, p<0.01) and vessel occlusion (Image 2).
We further conducted a single-center, open-label, single-arm interventional proof-of-concept study to assess the tolerability of 12 weeks of treatment with 10mg of EMPA as add-on therapy in eight patients with a prior PAH diagnosis. There were no treatment related adverse events during the study time course.
Conclusions: EMPA directly improved mitochondrial biogenesis and attenuated proliferation of MVECs from PAH patients and reversed PV remodeling in experimental PAH. EMPA treatment was feasible in patients with PAH. EMPA may serve as a new therapeutic option for PAH.
Methods and Results: Immunohistochemistry of pulmonary arteries revealed the expression of SGLT2 in the intima of PAH patients. Microvascular endothelial cells (MVECs) were isolated from lungs of control subjects (CTRL) and PAH patients and found intense expression of SGLT2 in PAH MVECs. Western blot showed significantly higher expression of SGLT2, while PGC-1α, a transcriptional coactivator known as a master regulator of mitochondrial biogenesis, was lower in PAH MVECs compared to CTRL (Image 1). PAH MVECs were treated with EMPA (1 μM) and observed a significant increase of PGC-1α and BMPR2-pSmad1/5/9. Similarly, knockdown of SGLT2 using siRNA resulted in enhanced expression of PGC-1α. Moreover, Seahorse analysis revealed increased oxygen consumption rate and reduced extracellular acidification rate by EMPA, suggesting that EMPA improved mitochondrial respiration. In addition, EMPA attenuated reactive oxygen species and proliferation of PAH MVECs, as revealed by MitoTracker analysis and MTT assay respectively.
For the in vivo study, Sprague-Dawley rats (120-180 gram) were injected with SU5416 (Week-0) and exposed to hypoxia for 3 weeks followed by normoxia. After randomization at Week-4, rats were treated with EMPA (300 mg/kg in chow, n=12) or placebo (PLAC, n=12). Catheterization and histological analysis were performed at Week-8. EMPA decreased PV resistance index (0.21±0.02 vs. 0.37±0.05 mmHg/mL/min/mm2, p=0.01), ameliorated intima thickening (21.0±7.5 vs. 40.0±7.8%, p<0.01) and vessel occlusion (Image 2).
We further conducted a single-center, open-label, single-arm interventional proof-of-concept study to assess the tolerability of 12 weeks of treatment with 10mg of EMPA as add-on therapy in eight patients with a prior PAH diagnosis. There were no treatment related adverse events during the study time course.
Conclusions: EMPA directly improved mitochondrial biogenesis and attenuated proliferation of MVECs from PAH patients and reversed PV remodeling in experimental PAH. EMPA treatment was feasible in patients with PAH. EMPA may serve as a new therapeutic option for PAH.
More abstracts on this topic:
Arl2bp and Mrps35 May Contribute to SOD2-Induced Increase in Endothelial Oxidative Phosphorylation
Brinck Teixeira Rayane, Xu Cynthia, Albro Jane, Sellke Frank, Abid Ruhul
A single-cell lung atlas of human pulmonary arterial hypertensionDai Zhiyu, Yi Dan, Zhao Hanqiu, Hong Jason, Fallon Michael