Final ID: MDP1708
Efficacy and Outcomes of Empagliflozin in Acute Coronary Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract Body (Do not enter title and authors here): Background:
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated mortality benefits in patients with heart failure (HF). Since acute coronary syndrome (ACS) is an increasingly prevalent cardiovascular condition that often leads to HF, SGLT2i might play a role in reducing mortality in these patients. Previous randomized controlled trials (RCTs) have demonstrated inconsistent efficacy of Empagliflozin, an SGLT2i, in patients with ACS.
Methods:
A comprehensive systematic literature search was conducted spanning the major bibliographic databases to retrieve RCTs comparing Empagliflozin to placebo in patients with ACS. Odds ratios (OR) and mean differences (MD) with 95% confidence intervals were pooled using the DerSimonian and Laird random-effects model with statistical significance set at p<0.05.
Results:
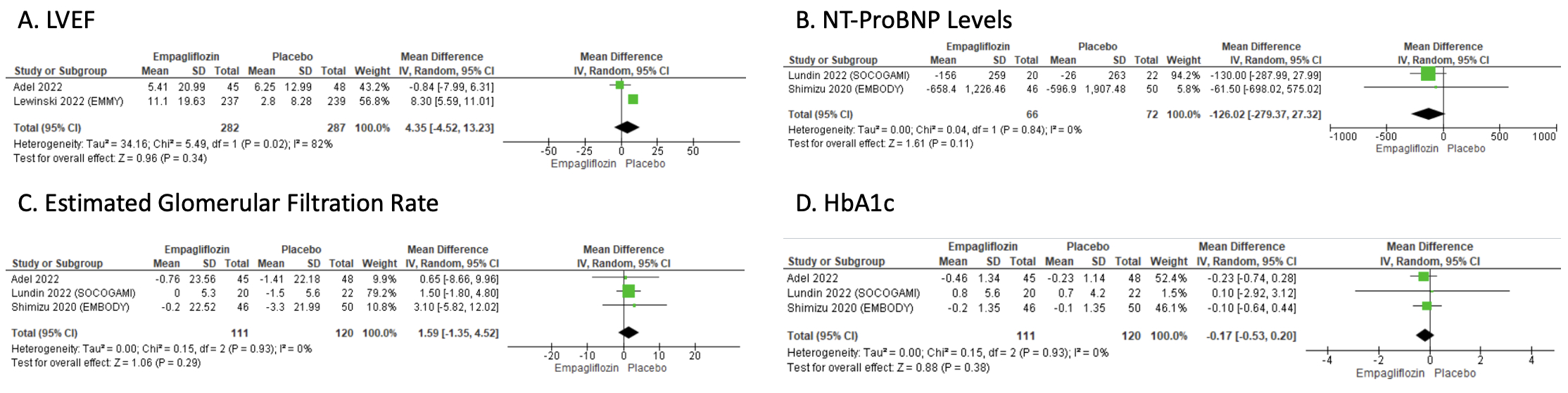
5 RCTs were included with 7229 ACS patients (3608 in the Empagliflozin group and 3621 in the placebo group). Empagliflozin treatment was not associated with a statistically significant difference in all-cause mortality [OR: 0.94; 95% CI: 0.76, 1.17; p=0.60], cardiovascular death [OR: 1.00; 95% CI: 0.79, 1.28; p=0.99], hospitalization for heart failure [OR: 0.86; 95% CI: 0.42, 1.74; p=0.67], serious adverse events [OR: 1.85; 95% CI: 0.29, 11.87; p=0.51], LVEF on follow-up [MD: 4.35; 95% CI: -4.52, 13.23; p=0.34], NT-ProBNP [MD: -126.02; 95% CI: -279.37, 27.32; p=0.11], estimated glomerular filtration rate [MD: 1.59; 95% CI: -1.35, 4.52; p=0.29], and HbA1c [MD: -0.17; 95% CI: -0.53, 0.20; p=0.38] compared to placebo.
Conclusion:
This study concludes no statistical difference in all-cause mortality, cardiovascular death, and hospitalization for heart failure with empagliflozin therapy in ACS patients compared to placebo, however, the largest study (EMPACT-MI) had a statistically significant difference in hospitalizations for HF. Further large multicentric RCTs are warranted to validate these findings.
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated mortality benefits in patients with heart failure (HF). Since acute coronary syndrome (ACS) is an increasingly prevalent cardiovascular condition that often leads to HF, SGLT2i might play a role in reducing mortality in these patients. Previous randomized controlled trials (RCTs) have demonstrated inconsistent efficacy of Empagliflozin, an SGLT2i, in patients with ACS.
Methods:
A comprehensive systematic literature search was conducted spanning the major bibliographic databases to retrieve RCTs comparing Empagliflozin to placebo in patients with ACS. Odds ratios (OR) and mean differences (MD) with 95% confidence intervals were pooled using the DerSimonian and Laird random-effects model with statistical significance set at p<0.05.
Results:
5 RCTs were included with 7229 ACS patients (3608 in the Empagliflozin group and 3621 in the placebo group). Empagliflozin treatment was not associated with a statistically significant difference in all-cause mortality [OR: 0.94; 95% CI: 0.76, 1.17; p=0.60], cardiovascular death [OR: 1.00; 95% CI: 0.79, 1.28; p=0.99], hospitalization for heart failure [OR: 0.86; 95% CI: 0.42, 1.74; p=0.67], serious adverse events [OR: 1.85; 95% CI: 0.29, 11.87; p=0.51], LVEF on follow-up [MD: 4.35; 95% CI: -4.52, 13.23; p=0.34], NT-ProBNP [MD: -126.02; 95% CI: -279.37, 27.32; p=0.11], estimated glomerular filtration rate [MD: 1.59; 95% CI: -1.35, 4.52; p=0.29], and HbA1c [MD: -0.17; 95% CI: -0.53, 0.20; p=0.38] compared to placebo.
Conclusion:
This study concludes no statistical difference in all-cause mortality, cardiovascular death, and hospitalization for heart failure with empagliflozin therapy in ACS patients compared to placebo, however, the largest study (EMPACT-MI) had a statistically significant difference in hospitalizations for HF. Further large multicentric RCTs are warranted to validate these findings.
More abstracts on this topic:
Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient Subgroups
Stern Lily, Fine Nowell, Maurer Mathew, Grogan Martha, Ambardekar Amrut, Grodin Justin, Soman Prem, Garcia-pavia Pablo, Chen Chris, Siddhanti Suresh, Tamby Jean-francois, Fox Jonathan
Acute Exposure to High PM2.5 Levels Increases the Risk of Late All-Cause Mortality in Patients with STEMIFathieh Sina, Tran Hao, Faour Amir, Pahn Reece, Long Mitchell, Tam Gladys, Figtree Gemma, Negishi Kazuaki, French John